Copper phthalocyanines, although generally regarded as classical organic pigments, exhibit outstanding technical performance and so could equally well be described as high-performance organic pigments. This section contains a brief survey of a range of the organic pigments, encompassing a wide variety of structural types, which have been developed in an attempt to match the properties of copper phthalocyanines in the yellow, orange, red and violet shades. They include two groups of azo pigments, carbonyl pigments of a variety of types and dioxazines. High — performance organic pigments are particularly suited to applications which require bright, intense colours and which at the same time place severe demands on the technical performance of pigments, such as the coatings applied to car bodies, referred to as automotive paints. They provide excellent durability, combined with good colour properties but they do tend to be rather expensive.
There are two classes of high-performance azo pigments: disazo condensation pigments and benzimidazolone azo pigments. The chemical structures of representative examples of these products are illustrated in Figure 9.2. Disazo condensation pigments, developed by Ciba Geigy, are a range of durable yellow, red, violet and brown products, with structures such as compound 213 (C. I. Pigment Red 166). These pigments derive their name, and also their relatively high cost, from the rather elaborate synthetic procedures involved in their manufacture which involves a condensation reaction (see Scheme 3.8, Chapter 3). Hoechst has developed a series of azo pigments that contain the benzimidazolone group, e. g.
|
Figure 9.2 Chemical structures of some high-performance azo pigments |
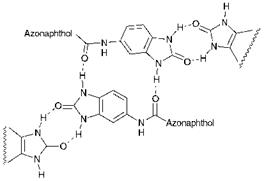
C. I. Pigment Red 183 (214), which range in shades from yellow to bluish — red and brown and exhibit excellent fastness properties. Their good stability to light and heat and their insolubility is attributed to extensive intermolecular association as a result of hydrogen bonding and dipolar forces in the crystal structure, as illustrated in Figure 9.3.
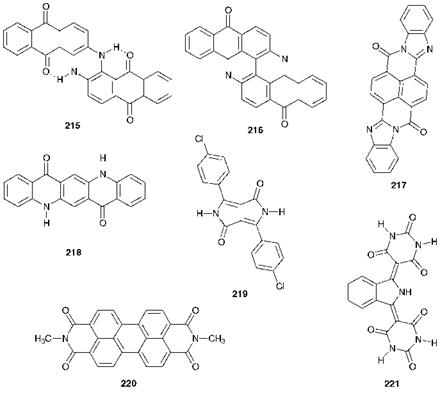
Carbonyl pigments of a variety of types may also be classed as high — performance products. These include some anthraquinones, quinac — ridones, perylenes, perinones, isoindolines and diketopyrrolopyrroles. The chemistry of these groups of colorant is discussed in Chapter 4. Some representative examples of chemical structures of important high-performance carbonyl pigments are illustrated in Figure 9.4.
A number of vat dyes developed originally for textile applications are suitable, after conversion into an appropriate pigmentary physical form, for use in many paint and plastics applications. Examples of these so — called vat pigments include the anthraquinones, Indanthrone Blue (215, C. I. Pigment Blue 60) and Flavanthrone Yellow (216, C. I. Pigment
|
Figure 9.3 Intermolecular association in the crystal structure of a benzimidazolone azo pigment |
|
Figure 9.4 Chemical structures of a range of high-performance carbonyl pigments |
Yellow 24) and the perinone 217 (C. I. Pigment Orange 43). Other high-performance carbonyl pigments include the quinacridone 218 (C. I. Pigment Violet 19), diketopyrrolopyrrole (DPP) pigments, such as C. I. Pigment Red 254 (219), perylenes, for example C. I. Pigment Red 179 (220) and isoindolines, such as C. I. Pigment Yellow 139 (221). The excellent lightfastness, solvent resistance and thermal stability of carbonyl pigments may be explained in many cases by intermolecular association in the solid state as a result of a combination of hydrogen bonding and dipolar forces, similar to that illustrated for the benzimidazolone azo pigments in Figure 9.4. A diagrammatic illustration of the intermolecular hydrogen bonding in the crystal lattice arrangement of quinacridone 218 is given in Figure 4.6, Chapter 4.
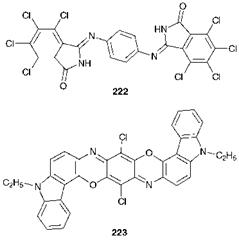
Other chemical types of high-performance organic pigments are exemplified by the tetrachloroisoindolinone 222 (C. I. Pigment Yellow 110) and the dioxazine 223 (Carbazole Violet, C. I. Pigment Violet 23). Considerable research has been carried out in an attempt to exploit the potential of metal complex chemistry to provide high-performance pig-
|
|
ments, particularly of yellow and red shades, to complement the colour range of the copper phthalocyanines (which cannot be extended outside blues and greens). A number of azo, azomethine and dioxime transition metal complex pigments have been obtained which show excellent lightfastness, solvent resistance and thermal stability. However, the products have achieved limited commercial success largely because the enhancement of the fastness properties of the organic ligand which results from complex formation is almost inevitably accompanied by a reduction in the brightness of the colour. This effect may be explained by a broadening of the absorption band as a result of overlap of the band due to n-n* transitions of the ligand with those due to transition metal d-d transitions or ligand-metal charge transfer transitions.
 23 декабря, 2015
23 декабря, 2015  Pokraskin
Pokraskin 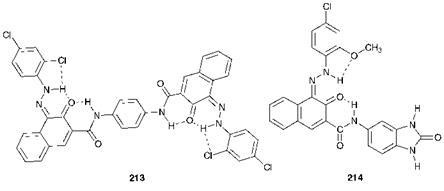
 Опубликовано в рубрике
Опубликовано в рубрике 