There has been considerable effort over many years aimed at the development of the means to convert solar energy into electrical energy. The potential advantages of solar power are obvious. It utilises a non-diminishing energy source and suffers little from the global environmental problems inherent in energy generation by nuclear fission or by the combustion of fossil fuels.
Dye-sensitised photocells, which utilise specific dyes for the purpose of solar energy conversion, have been investigated as an alternative to traditional silicon photocell technology. One type of construction for such a photocell is shown in Figure 10.6. The electrodes for the photocell are commonly thin films of metal (e. g. aluminium or gold) coated on to glass. At one electrode, there is a semiconducting interfacial layer of an inorganic substrate (e. g. fine particle-size or ‘nano-structured’ titanium dioxide), on to which the dye is adsorbed. Another important component of the photocell is an electrolyte, dissolved in an appropriate organic solvent and attracted into the intra-electrode space by capillary action. The electrolyte is commonly an iodine/tetraalkylammonium iodide mixture.
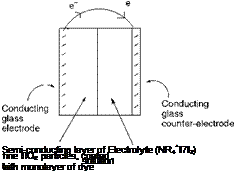 |
A mechanism which has been proposed for the operation of this type of photocell is illustrated in Figure 10.7, although it is not fully established in detail. In the proposed mechanism, it is suggested that absorption of light by the dye (or sensitiser, S) raises the dye to its first excited state S*. In the excited state, S* releases an electron into the conducting band of the titanium dioxide electrode, at the same time forming oxidised sensi — tiser, S + . At the counter-electrode, an electron is transferred to the
|
Figure 10.7 Proposed mechanism for the operation of a dye-sensitised photocell |
electrolyte and, mediated by the iodine/iodide equilibrium, the dye (S) is regenerated by reduction.
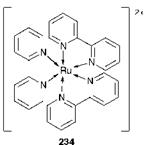
A dye which shows particular promise for this application is the octahedral ruthenium(ii) complex of 2,2′-bipyridyl (234). While this type of system appears to offer considerable potential as a means of solar energy conversion, the efficiency of the technology, at its current state of development, is significantly lower than that of traditional silicon photocells.
|
|
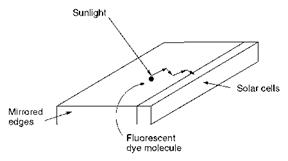
One of the deficiencies of conventional silicon cells used for solar energy conversion is their low efficiency at lower wavelengths in the UV and visible region. An approach to the solution of this problem has involved the development of dye-based solar collectors. These solar collectors utilise the ability of fluorescent dyes to absorb lower wavelengths of light and to re-emit the energy at higher wavelengths to which the photocells are more sensitive. In practice, solar collectors contain the fluorescent dye in a thin sheet of plastic, generally poly(methyl methacrylate), whose faces and edges are mirrored to channel the emitted radiation by internal reflection to the edge of the sheet containing the solar cell as illustrated in Figure 10.8. The requirements of
|
Figure 10.8 Design of a fluorescent dye-based solar collector |
dyes for this application are a high fluorescence quantum yield, compatibility with the plastic material, high lightfastness in the polymer matrix, little overlap of the absorption and emission spectra (generally associated with a large Stokes shift) and high purity. A wide range of dye classes have been examined for their suitability for application in solar collectors and the search for suitable dyes, especially for dyes with appropriate photostability, is continuing. Coumarins and perylenes are currently the most suitable classes of dye for these applications.
 10 января, 2016
10 января, 2016  Pokraskin
Pokraskin 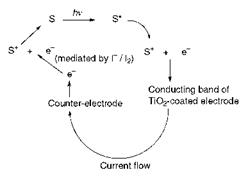
 Опубликовано в рубрике
Опубликовано в рубрике 