Liquid crystals, commonly referred to as the fourth state of matter, are materials which are intermediate in character between the solid and liquid states. Unlike normal isotropic liquids, they show some time — averaged positional orientation of the molecules, but they retain many of the properties of liquids, such as the ability to flow. In recent decades, liquid crystals have played an increasingly important part in our lives. Probably their most familiar application is in the information displays which provide the visual interface with microprocessor-controlled instrumentation. Liquid crystal displays have superseded more traditional display technology, such as light-emitting diodes and cathode ray tubes, for many appliances principally because of the advantages of visual appeal, low power consumption, and their ability to facilitate the miniaturisation of devices into which they are incorporated. They are encoun-
tered widely in many applications such as digital watches, calculators and instrument display panels in cars and aircraft.
Liquid crystal displays operate by utilising the ability to control ambient light to provide contrast, i. e. areas of dark and light, within the display. This is achieved as a result of a change in orientation of the liquid crystal molecules within certain sections of the display as an electric field is applied. In early devices a polariser was used to provide this contrast, but it was discovered that improved contrast could be provided by the use of certain specifically-designed dyes which are dissolved in the liquid crystal host material. These dichroic dyes are believed to align with the liquid crystal molecules and change direction with the application of the electric field, as illustrated in Figure 10.1, with a consequent change in colour intensity.
To explain the change in colour intensity with change in orientation of the dye with respect to the direction of incident light indicated by Figure
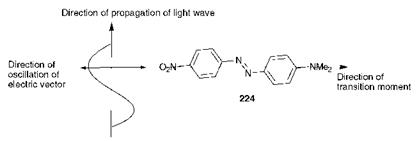
10.1, it is important to recognise that electronic excitation caused by light absorption is accompanied by a change in the polarity of the dye molecule. The electronic transition which occurs may be ascribed a transition dipole moment which has not only magnitude but also direction. The transition moment in aminoazobenzene dye 224, for example, is directed from the electron-releasing amino group to the electron-withdrawing nitro group, as illustrated Figure 10.2 (see Chapter 2 for a justification based on the application of the valence-bond approach to colour and constitution). It is of particular note that, in the case of dye 224, the transition moment is more or less aligned with the long axis of the molecule. For light absorption to occur, the electric vector of the incident light (perpendicular to the direction of propagation of light as illustrated
|
|
|
Figure 10.2 Orientation of an aminoazobenzene dye for maximum light absorption in a liquid crystal display |
in Figure 10.2) must oscillate in the same direction as the transition moment of the dye. If the transition moment of the dye is perpendicular to the direction of the light source, i. e. parallel to the electric vector, then maximum colour intensity will be achieved. If the molecule rotates through 90° when the field is switched, either on or off, then a change to minimum colour intensity will be observed.
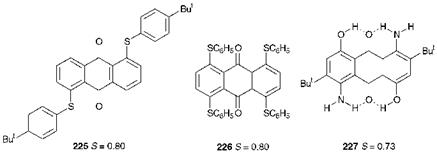
For application in liquid crystal displays, the dyes require good solubility in and compatibility with the liquid crystal host, and thus the dyes are invariably non-ionic. The dyes are also required to be of high purity and good lightfastness. Most important of all from the point of view of providing high contrast is the ability of the dye molecule to align, and switch, with the liquid crystal host. This ability may be quantified in terms of the order parameter, S, a measurable quantity (= 0 for nonalignment and = 1 for perfect alignment). The target value for a good liquid crystal display dye is S = 0.8. Generally, liquid crystal displays are required to produce black/white contrast, and thus to ensure that absorption occurs throughout the visible spectrum, a combination of yellow, red and blue dyes is required. Azo dyes, such as compound 224 are capable of providing only reasonable order parameters and also generally suffer from inadequate light stability. The dyes which most effectively satisfy the requirements for liquid crystal displays belong to the carbonyl chemical class. Anthraquinone dyes, such as the yellow dye 225, the red 226 and the blue 227 (illustrated in Figure 10.3) are especially suitable for these applications. It is commonly perceived that a rod-like molecular shape provides the required orientation behaviour for liquid crystal dyes. This feature is apparent in the structure of blue dye 227 and, additionally, the extensive intramolecular hydrogen bonding in this dye promotes good lightfastness. However, this type of molecular shape is not so apparent in the sulfur-containing anthraquinone dyes 225 and 226. These examples demonstrate that the nature of the interactions between the dye and the liquid crystal molecules in these displays is probably more complex than
|
Figure 10.3 Anthraquinone dyes used in liquid crystal displays |
that depicted in this simplistic approach and as yet must be considered as incompletely understood.
 5 января, 2016
5 января, 2016  Pokraskin
Pokraskin 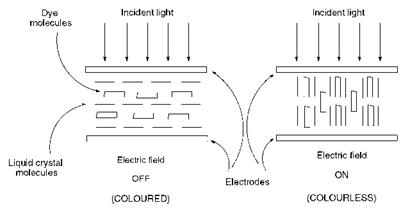
 Опубликовано в рубрике
Опубликовано в рубрике 