Over the last few decades, society has become increasingly sensitive towards the protection of the environment. We have developed a concern over a range of issues, amongst the most extensively debated being the destruction of the rain forests, global warming and the depletion of the ozone layer. Over this period, the chemical manufacturing industry has been faced with the need to address its responsibility towards a wide range of health, safety and environmental issues, and indeed, probably its most significant current challenges are associated with the requirement to satisfy the demands of stringent environmental controls. Concern about the potential adverse effects of the chemical industry on the environment is global nowadays, although the response in some parts of the world has been much faster and more intense than in others. The colour manufacturing industry represents a relatively small part of the overall chemical industry. It may be described, in general, as a small-volume, multiproduct industry and it has traditionally been highly innovative, constantly seeking to introduce new products. This has led to a requirement to address a wide range of toxicological and ecotoxicological issues both in the manufacture of dyes and pigments and in their application. This chapter seeks to present an overview of some of the more important general issues, without attempting to be comprehensive, because the diversity of chemical types and applications of dyes and pigments means that the range of individual issues is immense. A detailed discussion of the legislation and controls introduced by governments and regulatory agencies, the introduction of which has been a major factor in ensuring compliance of the industry with the most important issues, is outside the scope of this chapter, because of the complexity of the legislative detail, the fact that it varies substantially from country to country and because the situation is constantly evolving, so that information presented would quickly become out of date.
The colour manufacturer and user has a duty to address environmental and toxicological risks from a variety of points of view, including hazards in the workplace, exposure of the general public to the materials and the general effect on the environment. The level of risk from exposure to chemicals is clearly of prime concern for those handling materials in large quantities in the workplace, and this has required the introduction of modern work practices to minimise the exposure. The approach towards addressing the problems associated with exposure to potentially dangerous chemical substances, which has been adopted in most countries, commonly involves an evaluation of risk, including an assessment of the hazards presented by the various chemical species on the basis of the available toxicological data, and an assessment of exposure levels, and, from this evaluation, risk management strategies are developed.
Dyes and pigments are, by definition, highly visible materials. Thus, even minor releases into the environment may cause the appearance of colour, for example in open waters, which attracts the critical attention of the public and local authorities. There is thus a requirement on industry to minimise environmental release of colour, even in cases where a small but visible release might be considered as toxicologically rather innocuous. A major source of release of colour into the environment is associated with the incomplete exhaustion of dyes onto textile fibres from an aqueous dyeing process and the need to reduce the amount of residual dye in textile effluent has thus become a major concern in recent years. While this applies in principle to all application classes of textile dyes, the particular case of reactive dyes for wool and cellulose is of special interest because of the problem of dye hydrolysis, which competes with the dye-fibre reaction, the unfixed and hydrolysed dye inevitably appearing in the effluent. An extensive programme of research to address these problems has met with some success, leading to the development of more selective fibre-reactive systems and significantly improved processing conditions (Chapter 8). However, the development of a practical reactive dye system which is completely free of the problems associated with hydrolysis or incomplete dye-fibre reaction has so far proved elusive. An alternative approach to addressing the problem of colour in textile dyeing effluent has involved the development of effluent treatment methods to remove colour. These methods inevitably add to the cost of the overall process and some present the complication associated with the possible toxicity of degradation products. A number of chemical treatment methods for colour removal have been developed, of which the most successful involve oxidative degradation, for example using chlorine or ozone. Ozone treatment is particularly effective but is rather expensive. Physical treatments may also be used to remove colour, for example by the adsorption of dyes onto inert substrates such as activated charcoal, silica, cellulose derivatives or ion-exchange resins. Biological processes make use of the ability of living organisms to bind or degrade colour. Biodegradation processes offer the attraction of the potential for the decoloration of effluent with complete mineralisation of the organic materials present to carbon dioxide, water and inorganic ions such as nitrate, sulfate and chloride. However, the principal problem with the development of such a system is that synthetic dyes are generally xenobiotic, i. e. are not metabolised by the enzymes in the micro-organisms present naturally in waters, and the solution may require the development of micro-organisms cultured specifically for the purpose of metabolising synthetic dyes. The problems associated with textile effluent are not restricted to the dyes themselves. There are also issues associated with the presence of traces of heavy metals, notably the excess chromium used in the chrome mordanting of wool, the high levels of inorganic salts and other auxiliaries required by certain textile dyeing processes, and the use of reducing agents in the case of sulfur dyes. Each specific process presents its own set of environmental issues which are required to be addressed by the development of new products, process improvements or effluent treatment methods (Chapter 7). For pigments, because of their insolubility and the particular way they are used, the loss into the environment is much less than with textile dyes.
The importance of being aware of the potential adverse effects of exposure to chemicals on our health is self-evident. Toxic effects may be categorised in a number of ways. Acute toxicity refers to the effects of short-term exposure to a substance, for example in a single oral administration. It is relatively reassuring that studies of textile dyes suggest that there is little evidence for acute oral toxicity, and that most show little or no toxicological effect. Only in the cases of a few cationic dyes and some disazo dyes have some significant toxic effects been suggested. Pigments and vat dyes generally show remarkably low acute toxicity. This is generally attributed to their extremely low solubility in body fluids, which means that they are capable of passing through the digestive system without absorption into the bloodstream.
Chronic toxicity refers to the effect of regular exposure over a prolonged period of time. Arguably the most severe chronic toxicological effect is the potential to induce cancer. There has been considerable concern in recent years over the potential carcinogenicity of certain azo dyes, which centres on the possibility of metabolism of the dyes by reductive cleavage of the azo group to give the aromatic amines from which they are derived synthetically. A number of aromatic amines which have been used in colour manufacture are recognised carcinogens. In particular, it is well established that benzidine (252a) and 2-naph- thylamine (253) are potent human carcinogens. Epidemiological studies carried out in the first half of the 20th century demonstrated a pronounced increase in the incidence of bladder cancer in workmen employed in the dye manufacturing industry who had been exposed to these two amines. As a consequence, responsible manufacturers in the Western world discontinued the manufacture of dyes from these amines, although the manufacture continued in certain other parts of the world. It has emerged subsequently that benzidine-derived azo dyes, such as Congo Red, C. I. Direct Red 1 (254), may also present a carcinogenic risk, an effect attributed to the metabolism of the dyes to benzidine by enzymatic reduction. Certain European countries have examined the cancer-causing potential of azo dyes critically, by focusing on the amines which would be released if reductive cleavage of the azo group were to take place. In Germany, for example, the approach has been to ban the manufacture and importation of all dyes derived from a list of twenty aromatic amines believed to be animal carcinogens. On the other hand, it is by no means true that all azo dyes should be considered as potential carcinogens. Studies of structure-carcinogenicity relationships in azo dyes have demonstrated, for example, that when the amine produced by reductive cleavage of the azo group contains a sulfonic acid group there is little or no carcinogenic risk. Azo pigments are considered to present a considerably reduced risk compared with azo dyes, so that, for example, they are not included in the German legislation. 3,3′-Dichlorobenzidine (DCB), 252b, is on the German list of carcinogenic amines, yet it continues to be used in the manufacture of a series of important commercial disazo pigments (Diarylide Yellows and Oranges, see Chapter 9), since there does not appear to be strong experimental evidence that these pigments are metabolised to DCB. However, there is evidence that the
|
|
Diarylide pigments may cleave thermally with prolonged heating above 240 °C to give a series of decomposition products which include DCB. Since this evidence emerged, there has been a decline in the use of these pigments in applications where high temperatures are likely to be encountered, for example in thermoplastics. Exposure to some types of dyes can give rise to rather less severe chronic toxicity effects, for example contact dermatitis and, especially for certain reactive dyes, respiratory sensitisation.
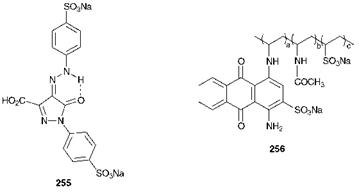
There are a number of interesting issues associated with the colours present in foods, and their influence on our health. Most foods contain natural colouring materials and, in addition, it is common to add colour to enhance the appeal of some foods to the consumer. There is growing evidence that certain natural colorants, for example the carotenoids present in fresh fruit and vegetables (Chapter 6), are of therapeutic value as a consequence of their anti-oxidant properties, which, it is suggested, may provide protection against cancer. There is an increasing tendency to use natural dyes as food additives, but synthetic dyes are more commonly used as they offer the advantage of higher colour strength and lower cost. A relatively restricted range of synthetic dyes are considered acceptable for use as food colorants, mainly because of the stringent toxicological requirements. An important example of a synthetic food colorant is the monazo dye Tartrazine, C. I. Food Yellow 23 (255). It is of note that reductive enzymatic cleavage of the azo group in this dye would lead to sulfonated aromatic amines, which are considered to present little toxicological risk. An interesting development in food colours is a range of polymeric dyes, such as the anthraquinone dye 256. It is considered that these dyes present little risk because their high molecular weight means that they do not penetrate through the membranes of the gastrointestinal tract.
|
|
The extension of the use of natural dyes into an even wider range of applications, including textiles and leather, as alternatives to synthetic dyes would seem superficially to offer attractive possibilities. In the current debate on the issues involved, the range of opinions expressed is extremely wide. There are environmental arguments which support the use of natural dyes, as this is seen to be exploiting renewable resources thus making a contribution towards environmental sustainability, at the same time presenting minimal risk to human health. There are, however, a number of counter-arguments. Natural dyes are generally more expensive and show inferior technical performance compared with synthetic dyes. In addition, the large-scale production and use of natural dyes might well introduce environmental problems, for example the need to cultivate large areas of land and the likelihood that the coloration processes would not necessarily be free from pollution problems. A possible outcome might be a renewed interest in natural dye research towards improved product performance, production methods, and application processes, and the emergence of certain niche markets which take advantage of the positive environmental perception associated with natural colours.
The issue of the so-called ‘heavy metals’ is of some concern within the chemical industry. While the term ‘heavy metal’ might have originally been derived on the basis of density, this property alone does not necessarily relate directly to toxicity or environmental behaviour. In fact, certain metals, for example iron, zinc, manganese, copper, chromium, molybdenum and cobalt, might technically be described as ‘heavy’, yet they are essential for life. The term has come to be used to represent those metals which are regarded as detrimental to the environment when a certain concentration is exceeded. The elements mercury, cadmium and lead occupy a special position because the concentration at which they begin to become detrimental to the health of organisms is very low. In addition, when absorbed in excessive amounts they may accumulate and can cause chronic health problems. Mercury is not a constituent of any significant commercial colorant. However, one important use of mercury derivatives is as a catalyst in the sulfonation of anthraquinone, an essential first step in the synthesis of some anthraquinone dyes (Chapter 4). Special care is required to ensure that mercury is not released into the environment in the effluent from such processes. Inorganic pigments based on lead (lead chromates and molybdates) and cadmium (cadmium sulfides and sulfoselenides) are still used commercially (Chapter 9). Their use nowadays is restricted significantly by a series of voluntary codes of practice, reinforced by legislation in certain cases, for example in toy finishes, graphic instruments and food contact applications where ingestion is a possibility. Cadmium pigments are used mainly in the coloration of certain engineering plastic materials which require very high temperature processing conditions. At present, completely satisfactory substitutes for such applications are not available, especially in terms of thermal and chemical stability. Lead chromates continue to be used, mainly in coatings, because they remain by far the most cost-effective, high durability yellow and orange pigments. Furthermore it may be argued that lead and cadmium pigments do not present a major health hazard or environmental risk, because of their extreme insolubility. Nevertheless, it seems likely that the progressive replacement of these products by more acceptable inorganic and organic pigments will continue in an increasing range of applications. Chromium(vi) is also considered to be highly toxic. In addition to its presence in lead chromate pigments, dichromates are used in the dyeing of wool by the chrome mordanting process (Chapter 7). This process remains of some importance as a cost-effective means of producing deep colours, which are fast to light and washing, on wool. The continuing acceptability of this dyeing method owes much to recent process improvements, for example the development of dyeing auxiliaries and procedures which minimise the level of residual excess chromium in textile dye effluent. There are toxicological and environmental issues associated with a number of other metals used to a certain extent in colorants, for example barium, manganese, cobalt, nickel, copper and zinc, but at present their use remains acceptable.
Certain organic chlorine derivatives, most notably the poly — chlorobiphenyls (PCBs), are known to be highly toxic and give rise to considerable environmental concerns. The potential to form PCBs in trace amounts has been noted in the manufacture of certain colorants, for example when aromatic chloro compounds are used either as reactants or solvents. In such situations, the industry has been required to respond by developing processes either to eliminate PCB formation or to ensure that the levels are below the rigorous limits set by legislation. Some environmental agencies and activist groups have taken a more extreme view and advocated a complete ban on all chlorinated organic chemicals. Such a ban would have a major effect on the colorant industry because of the large number of organic dyes and pigments which contain chlorine substituents. So far, legislation to this effect has not been introduced. Indeed, any move in such a direction would be an over-reaction, since there is little evidence that the mere presence of chlorine in a molecule means that it poses an environmental risk.
Environmental arguments are highly emotive. There are many reasons why the public has become sensitive to environmental issues and, to a certain extent, suspicious of the attitudes of the chemical industry in this respect. The industry has made errors in the past, some with severe human and environmental consequences, and has had to examine its conscience on occasions for having paid inadequate regard to health, safety and environmental issues. However, there is a point of view that the pendulum may have swung to the opposite extreme, so that, for example, arguments based on perception, rather than solid scientific evidence, often win the day. The need for rigorous experimentation to address toxicological and environmental concerns has acted as a deterrent to innovation in terms of the introduction of new products, in view of the considerable expense required in testing before a product may be introduced into the marketplace. We should not lose sight of the fact that the main reason for the existence of the synthetic colour industry is that dyes and pigments enhance our environment, by bringing attractive colours into our lives. It would indeed be a dull world if, for example, television, movies, photography and magazines essentially only provided black and white images, and if automobiles were available in ‘any colour we like provided it’s black!’ The days when such a situation existed are not so long ago in relative historical terms. Equally, it is vital that those industries involved in the manufacture and application of colour should continue to be sensitive to any potential adverse effect on the environment in its wider sense, and respond accordingly. A balanced approach will ensure protection of the environment and allow an innovative colorant industry to thrive in the 21st century.
 25 января, 2016
25 января, 2016  Pokraskin
Pokraskin 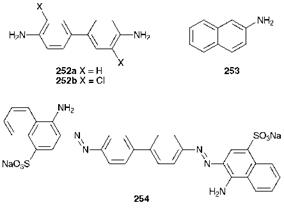
 Опубликовано в рубрике
Опубликовано в рубрике 