Chemichromism is a term which has been introduced to describe a change in colour, caused by a chemical conversion, induced by some external stimulus, for example exposure to light, heat or electric current. Examples of these phenomena have been well known for many years but, for many traditional applications such as dyes applied to textiles, colour changes, for example when exposed to light, were regarded as a nuisance and highly undesirable. As time has progressed, and as potential niche applications have been recognised, there has been a resurgence of interest in dyes which exhibit phenomena of this type, especially when the effects are reversible and controllable.
Probably the most familar and longest-established use of reversible colour change chemistry is found in titration indicators. The best known of these compounds are the indicators used traditionally to detect the end-point in an acid-base titration, as a result of a colour change, which takes place within a specific narrow pH range. As an example, the azo dye, methyl orange (241) changes from orange to red in the pH range 3-4, due to conversion into the protonated species (242) (Scheme 10.1). Other types of sensor which make use of specific colour changes include redox indicators, which show a reversible colour change as a result of electron transfer reactions, and chromoionophores, which give rise to specific colour changes when they react with certain metal ions and thus may be used as sensitive reagents for metal ion detection.
|
Scheme 10.1 Colour change in the protonation of methyl orange (241), an acid-base indicator |
 17 января, 2016
17 января, 2016  Pokraskin
Pokraskin 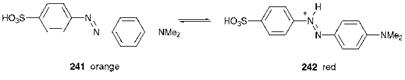
 Опубликовано в рубрике
Опубликовано в рубрике 