There are a number of carbonyl colorant systems, which offer particular advantages as pigments for applications, such as automotive paints, which demand extremely high performance in terms of colouristics and fastness to light, heat and solvents. These carbonyl pigments are diverse structurally and include the quinacridones, diketopyrrolopyrroles, pery — lenes, perinones and anthraquinones and the first four of these types are discussed in this section. The use of anthraquinones as vat pigments has been discussed in a previous section. Commonly, these pigments owe their outstanding fastness properties to intermolecular hydrogen-bonding involving the carbonyl groups.
The quinacridones constitute one of the most important chromophoric systems developed for pigment applications since the phthalocyanines (Chapter 5). Linear trans quinacridones were first discovered in 1935, but their potential as pigments was not realised until the late 1950s, when they were introduced commercially by DuPont. The pigments offer outstandingfastness properties, similar to those of copper phthalocyanine, in the orange, red and violet shade areas. Structurally, quinacridones consist of a system of five fused alternate benzene and 4-pyridone rings. A number of geometrical arrangements of such a system are possible, e. g. 65, 66 and 67, but the outstanding technical properties are only given by compounds which possess the linear trans arrangement (65). Compounds 65a-c are the products of most significant commercial importance.
At first sight, it may seem somewhat surprising that such small molecules should provide such a high degree of thermal and chemical stability and insolubility. These properties have been explained, however, by strong two-dimensional molecular association due to hydrogen bonding
|
|
in the crystal structure between N-H and C=O groups, as illustrated in Figure 4.6. There is considerable evidence from related derivatives for the importance of intermolecular H-bonding in determining the properties of the quinacridones. For example, the inferior properties of the angular quinacridone 67 and the 4,11-dichloro derivative 65d may be attributed to steric and geometric constraints, which reduce the efficiency of the hydrogen bonding. In addition, the X, X-dimethyl derivatives, in which no H-bonding is possible, are reported to be soluble in organic solvents. A further factor which will play a part in the technical performance of quinacridone pigments is the strong dipolar nature of the pyridone rings that arises from a major contribution from resonance forms such as 68, leading to strong intermolecular dipolar association throughout the crystal structure.
The origin of the colour of quinacridone pigments provides a good example of the influence of crystal lattice effects, which have not yet been explained fully in fundamental terms. It is interesting that in solution the quinacridones exhibit only weak yellow to orange colours. The intense red to violet colours of the pigments in the solid state are therefore presumably due to interactions between molecules in the crystal lattice. The quinacridones show polymorphism and this has a profound effect on the colour of the pigments. The parent compound 65a exists in three distinct polymorphic modifications each one with its own characteristic X-ray powder diffraction pattern. Two of these forms, the a — and ^- modifications, are red, while the у-form is violet. The a-form is the least stable to polymorphic change and has not been commercialised.
There is little doubt that one of the most significant developments in
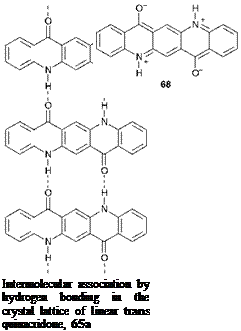 |
Figure 4.6
organic pigments in recent times is the discovery and commercialisation of pigments based on the 1,4-diketopyrrolo[3,4-c]pyrrole (DPP) system. The essential structural feature of this group of pigments, of which C. I. Pigment Red 254 (69) is a representative commercial example, are the two fused five-membered ketopyrrole rings. DPP pigments, by appropriate substituent variation, are capable of providing orange through red to bluish-violet shades. In particular, the DPP pigments which have gained most prominence provide brilliant saturated red shades of outstanding durability for automotive paint applications. In addition, their excellent thermal stability means that they are of considerable interest for the pigmentation of plastics. The pigments are, like the quinacridones, remarkable in producing such an excellent range of fastness properties from such small molecules. X-ray structural analysis has demonstrated that this is due to the strong intermolecular forces, due to hydrogen bonding and dipolar interactions, which exist throughout the crystal structure, similar to those involved with the quinacridones.
|
|
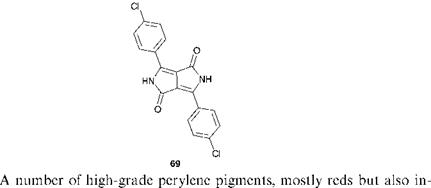
eluding blacks, are of importance. The pigments may be represented by the general structure 70, in which the imide nitrogen substituents, R, may be alkyl or aryl groups. An interesting observation in the perylene series is that small structural changes in the side-chain can lead to quite profound colour differences. The N, N’-dimethyl compound, for example, is red while the corresponding diethyl derivative is black. X-ray diffraction studies have now been applied to an extensive range of perylenes in an attempt to characterise the effect of differences in the crystal lattice structure on the light absorption properties of the pigments, a phenomenon known as crystallochromy. As an example, the A, A’-dimethyl compound consists of a parallel arrangement of molecules in stacks, whereas in the A, A’-diethyl compound the molecules are in stacks twisted with respect to one another with considerably more overlapping of the perylene ring systems in neighbouring molecules. Some perylenes with bulky substituents on the imide nitrogens are useful highly efficient and stable fluorescent dyes. They are suitable for use in demanding applications such as in solar energy collectors. The strong fluorescence of these dyes has been attributed to the structural rigidity of the molecules. A group of carbonyl pigments, which are structurally related to the perylenes are the perinones. Two isomeric perinone pigments are manufactured, C. I. Pigment Orange 43, 71, the trans isomer, and C. I. Pigment Red 194,72, the cis isomer, the former being an especially important high performance product particularly for plastics applications.
 26 сентября, 2015
26 сентября, 2015  Pokraskin
Pokraskin 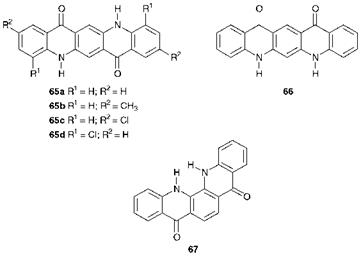
 Опубликовано в рубрике
Опубликовано в рубрике 