There is no doubt that the major weakness of the reactive dyeing process is the hydrolysis reaction and the consequent need for a wash-off process. The extent to which dye hydrolysis takes place in competition with dye-fibre reaction varies quite markedly within the range 10-40% depending upon the system in question. A considerable amount of research has therefore been devoted to the search for reactive dyes with improved fixation. The most successful approach to addressing this issue has involved the development of dyes with more than one fibre-reactive group in the molecule, which statistically improves the chances of dye-fibre bond formation. Examples of products of this type are the Procion H-E
|
Scheme 8.3 Reaction of a-bromoacrylamido reactive dyes with wool |
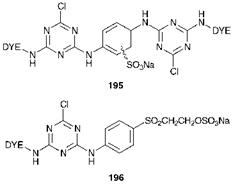
range which contain two monochlorotriazinyl reactive groups, for example as represented by general structure 195 and some dyes of the Remazol range which contain two ^-sulfatoethylsulfone groups. These are examples of homo-bisfunctional dyes. Another approach utilises two different types of reactive groups, the so-called hetero-bisfunctional dyes, as for example structure 196 which contains both a monochlorotriazinyl and a ^-sulfatoethylsulfone group. This particular class of reactive dye, because of the differing reactivities of the two groups, can offer greater flexibility in performance, for example reducing sensitivity to temperature and to pH variation. Although these bifunctional reactive dyes offer a significantly improved degree of fixation, the development of a practical reactive dye system which is completely free of the problems associated with hydrolysis or incomplete dye-fibre reaction remains an important target for dye chemists, and one which so far has proved elusive in spite of many years of research effort.
|
|
 17 ноября, 2015
17 ноября, 2015  Pokraskin
Pokraskin 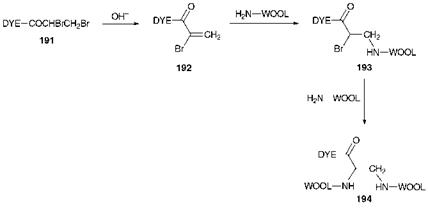
 Опубликовано в рубрике
Опубликовано в рубрике 