Arylcarbonium ion colorants were historically the first group of synthetic dyes developed for textile applications. In fact, Mauveine, the first commercial synthetic dye, belonged to this group (Chapter 1). The majority of the arylcarbonium ion colorants still in use today were discovered in the late 19th and early 20th centuries. As a group they are used considerably less than in former times, but many are still of some importance, particularly for use as basic (cationic) dyes for the coloration of acrylic fibres and paper, and as pigments. Structurally, they are closely related to the polymethine dyes, especially the cyanine types, and they tend to show similar properties. For example, they provide extremely intense, bright colours, covering virtually the complete shade range, but they are generally inferior in technical properties compared with the azo, carbonyl and phthalocyanine chemical classes and as a result their importance has declined over the years.
|
|
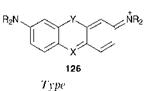
Arylcarbonium ion dyes encompass a diversity of structural types. Most of the dyes are cationic but there are some neutral and anionic derivatives. The best-known arylcarbonium ion dyes are the diaryl — methines, such as Auramine O, C. I. Basic Yellow 2 (124) and the triarylmethines, the simplest of which is Malachite Green, C. I. Basic Green 4, 125. The essential structural feature of these two groups is a central carbon atom attached to either two or three aromatic rings. Of these two groups, the triarylmethines are generally the most stable and thus the most useful. Commonly, they are referred to as triarylmethanes (or triphenylmethanes), but the name triarylmethines more correctly indicates that the carbon atom to which the aromatic rings are attached is sp2, rather than sp3, hybridised. Aza analogues of these dyes in which the central carbon is replaced by a nitrogen atom are also conveniently included in this class. There are also a number of derivatives obtained by bridging the di — and triarylmethines and their aza analogues across the ortho-ortho’ positions of two of the aromatic rings with a heteroatom. Examples of these types of heterocyclic systems, which may be represented by the general structure 126, are illustrated in Table 6.1.
Table 6.1 Heterocyclic arylcarbonium ion dyes and their aza analogues
|
|
|
-C(Ar)= |
-O- |
Xanthene |
|
-C(Ar)- |
-S- |
Thioxanthene |
|
-C(Ar)- |
-NR- |
Acridine |
|
-N= |
-O- |
Oxazine |
|
-N і |
-S- |
Thiazine |
|
-N — |
-NR- |
Azine |
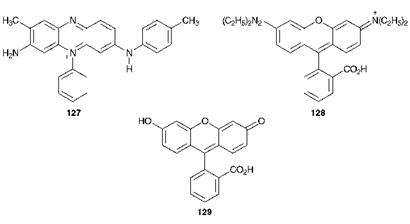
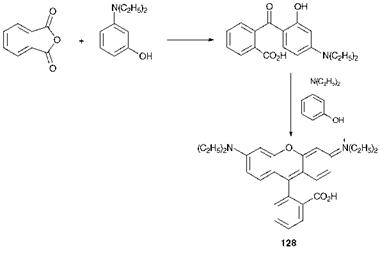
Mauveine, the original synthetic dye, was of the azine type, its principal component being compound 127. This particular group of dyes are now essentially only of historic interest. Xanthene dyes, such as Rhodamine B, C. I. Basic Violet 10 (128) and fluorescein (129), are relatively inexpensive dyes which are notable for their strong fluorescence, a feature which has been attributed to their structural rigidity. They are used in a wide range of applications, including the detection of defects in engineered articles and in the tracking of water currents. Rhodamines are also used in dye lasers (Chapter 10)
|
|
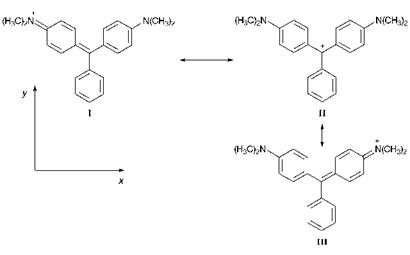
The valence-bond (resonance) description of the triphenylmethine dye Malachite Green (125) is illustrated in Figure 6.5. Comparison with Figure 6.4 reveals their structural similarity compared with cyanine dyes. Formally, the dye contains a carbonium ion centre, as a result of a contribution from resonance form II. The molecule is stabilised by resonance that involves delocalisation of the positive charge on to the p-amino
|
Figure 6.5 Valence-bond (resonance) approach to Malachite Green 125 |
nitrogen atoms as illustrated by forms I and III. Because of the steric constraints imposed by the presence of the three rings, triarylmethine dyes cannot adopt a planar conformation. The three rings are twisted out of the molecular plane, adopting a shape like a three-bladed propeller. Malachite Green shows two absorption bands in the visible region with Лтах values of 621 and 428 nm. Hence, its observed green colour is due to the addition ofblue and yellow components. The long wavelength band is polarised along the x-axis and the short wavelength band along the y-axis.
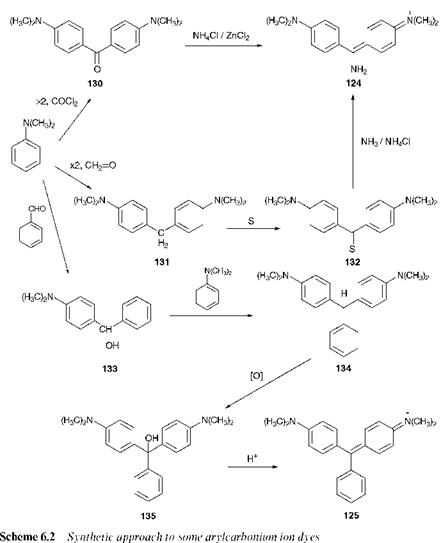
The synthesis of arylcarbonium ion dyes and pigments generally follow a similar set of principles. A few selected examples are shown in Scheme 6.2 to illustrate these principles. Essentially, the molecules are constructed from aromatic substitution reactions. In general, a C electrophile, for example phosgene (COCl2), formaldehyde, chloroform or carbon tetrachloride, reacts with an aromatic system which is activated to electrophilic attack by the presence of a strongly electron-releasing group such as the amino (primary, secondary or tertiary) or hydroxy group. Symmetrical diarylmethines and triarylmethines may be synthesised in one operation, for example by reaction of one mole of phosgene with either two or three moles of the appropriate aromatic compound. Depending on the particular electrophile used, an oxidation may be required at some point in the reaction sequence to generate the final product. Two methods of synthesis of the diarylmethine Auramine O (124) are shown in Scheme 6.2. Formerly, this yellow dye was prepared from Mischler’s Ketone (130), which may be obtained from the reaction of dimethylani-
|
|
line (2 mol) with phosgene (1 mol). In the currently preferred method, dimethylaniline (2 mol) is reacted with formaldehyde (1 mol) to give the diaryl compound 131. This compound is then heated with sulfur and ammonium chloride in a stream of ammonia at 200 °C. The dye 124 is formed via the thiobenzophenone 132 as an intermediate. The synthesis of Malachite Green, 125, is given in Scheme 6.2 to illustrate how an unsymmetrical triarylmethine derivative may be prepared. Dimethylani — line is reacted with benzaldehyde under acidic conditions to give the alcohol 133. This compound is then treated with a further equivalent of
|
Scheme 6.3 Synthesis of Rhodamine B, 128 |
dimethylaniline to give the leuco base 134, which is subsequently oxidised to the carbinol base 135. Acidification of this compound leads to Malachite Green (125). For many years, lead dioxide (PbO2) was the agent of choice for oxidation reactions of this type. Lead-free processes, for example using air or chloranil in the presence of various transition metal catalysts, are now preferred for toxicological and environmental reasons. By using different aromatic aldehydes and aromatic amines as starting materials, this method may be adapted to produce a wide range of triarylmethine dyes.
As an example of a heterocyclic arylcarbonium ion dye, the method of synthesis of Rhodamine B (128) is shown in Scheme 6.3. The starting materials in this case are phthalic anhydride and 3-N, N-die — thylaminophenol.
Each of the products whose synthesis is illustrated in Schemes 6.2 and 6.3 are coloured cationic species. When the counter-anion is chloride, the products are water-soluble and useful as basic dyes for acrylic fibres. Precipitation of cationic dyes of these types from aqueous solution using large polymeric counter-anions, notably phosphomolybdates, phos — photungstates and phosphomolybdotungstates leads to a range of highly insoluble red, violet, blue and green pigments. These pigments exhibit high brilliance and intensity of colour and high transparency, and are thus well suited to some printing ink applications.
|
|
 19 октября, 2015
19 октября, 2015  Pokraskin
Pokraskin 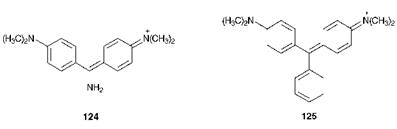
 Опубликовано в рубрике
Опубликовано в рубрике 