A common arrangement of the carbonyl groups in coloured molecules gives rise to a group of compounds known as quinones. These may be defined as cyclohexadienediones, i. e. compounds containing two ketone carbonyl groups and two double bonds in a six-membered ring. The simplest quinones are o — and p-benzoquinones, 49 and 50 respectively. Derivatives of the benzoquinones and of the naphtho-1,4-quinone system 51 are of only minor interest as colouring materials. This is probably due, at least in part, to the instability which results from the presence of an alkene-type double bond. By far the most important quinone colorants are the anthraquinones, or more correctly anthra-9,10-quinones. Anthraquinones, as demonstrated by the parent compound 52, contain a characteristic system of three linear fused six-membered rings in which the carbonyl groups are in the central ring and the two outer rings are fully aromatic. Anthraquinone colorants can give rise to the complete
|
|
range of hues. However, within any particular application class, they are often more important as violets, blues and greens, thus complementing the azo chemical class which generally provides the most important yellows, oranges and reds. They commonly give brighter colours than azo dyes, but the colours are often weaker, one reason why they are in general less cost-effective than azo dyes. Anthraquinone dyes provide excellent lightfastness properties, generally superior to azo dyes.
There is a wide diversity of chemical structures of anthraquinone colorants. Many anthraquinone dyes are found in nature, perhaps the best known being alizarin, 1,2-dihydroxyanthraquinone, the principal constituent of madder (see Chapter 1). These natural anthraquinone dyes are no longer of significant commercial importance. Many of the current commercial range of synthetic anthraquinone dyes are simply substituted derivatives of the anthraquinone system. For example, a number of the most important red and blue disperse dyes for application to polyester fibres are simple non-ionic anthraquinone molecules, containing substituents such as amino, hydroxy and methoxy, and a number of sul — fonated derivatives are commonly used as acid dyes for wool.
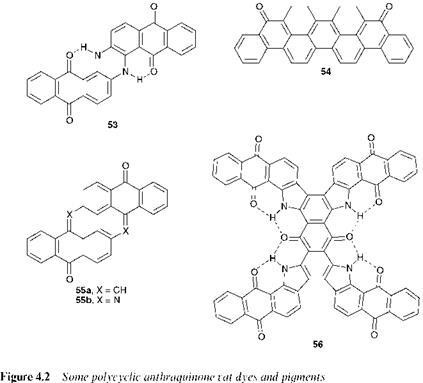
There are a large number of anthraquinones which are structurally more complex and polycyclic in nature. In this book, provided that the anthraquinone nucleus is recognisable somewhere in the structure, the colorant is classed as a member of the anthraquinone class, although some texts consider these annellated derivatives separately. These polycyclic anthraquinones belong almost invariably to the vat dye textile application class where their large extended planar structure is an important feature for their application (see Chapter 7). There are around 200 different anthraquinonoid vat dyes in use commercially, of widely different structural types, and covering the entire spectrum from yellow through blue to black. Figure 4.2 shows a selection of these compounds, including both carbocyclic and heterocyclic types. The examples presented are indanthrone (53), C. I. Vat Blue 4, violanthrone (54), C. I. Vat Blue 20, pyranthrone (55a), C. I. Vat Orange 9, flavanthrone (55b), C. I. Vat Yellow 1 and C. I. Vat Green 8, (56), a derivative containing 19 fused rings. While the anthraquinone chromophoric grouping is second only in importance to the azo chromophore in the chemistry of textile dyes, it is of considerably less importance in pigments. This is probably due to the
|
|
fact that the traditional role of anthraquinones in many dye application classes, which is to provide lightfast blues and greens, is more successfully adopted by the phthalocyanines (Chapter 5) in the case of pigments. However, the insolubility and generally good fastness properties of vat dyes stimulated considerable effort into the selection of suitable examples of the colorants for use, after conversion into the appropriate physical form, as pigments for paint, printing ink and plastics applications. Of the known vat dyes, only about 25 have been fully converted into pigment use, and less than this number are of real commercial significance. The range of anthraquinone pigments includes some of the longest-established vat dyes, notably indanthrone (53, C. I. Pigment Blue 60) together with some of its halogenated derivatives, and flavanthrone (55b, C. I. Pigment Yellow 24).
Anthraquinone (52) is only weakly coloured, its strongest absorption being in the UV region (Ятах 325 nm). The UV/visible spectral data for a series of substituted anthraquinones, 52a-h, are given in Table 4.1 and these illustrate the effect of the substituent pattern on the colour. The introduction of simple electron-releasing groups, commonly amino or
|
Table 4.1 Absorption maxima for some substituted anthraquinones
|
hydroxy, into the anthraquinone nucleus gives rise to a bathochromic shift which is dependent on the number and position of the electronreleasing group(s) and their relative strengths (OH < NH2 < NHAr). They are thus typical donor-acceptor systems, with the carbonyl groups as the acceptors and the electron-releasing auxochromes as the donors. By choice of an appropriate substitution pattern, dyes are obtained which may absorb in any desired region of the visible spectrum. From the data, it is clear that the electron-releasing groups exert their maximum bathochromic effect in the а-positions (1-, 4-, 5-, 😎 rather than the ^-positions (2-, 3-, 6-, 7-). This is one reason why substitution in a — positions is preferred in anthraquinone dyes. In addition, а-substituents give dyes with higher molar extinction coefficients and, very importantly, they enhance technical performance, especially lightfastness, by virtue of their participation in intramolecular hydrogen bonding with the carbonyl groups. The 1,4-substitution pattern is particularly significant in providing blue dyes of commercial importance in a variety of textile dye application classes (Chapter 7).
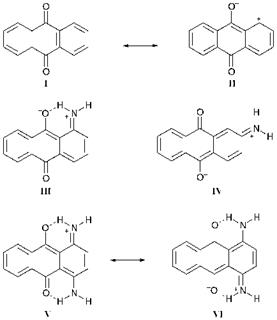
The effect of substituents on colour in substituted anthraquinones may be rationalised using the valence-bond (resonance) approach, in the same way as has been presented previously for a series of azo dyes (see Chapter 2 for details). For the purpose of explaining the colour of the dyes, it is assumed that the ground electronic state of the dye most closely resembles the most stable resonance forms, the normal Kekule-type structures, and that the first excited state of the dye more closely resembles the less stable, charge-separated forms. Some relevant resonance forms for anthraquinones 52, 52c, 52d and 52f are illustrated in Figure 4.3. The ground state of the parent compound 52 is assumed to resemble closely structures such as I, while charge-separated forms, such as structure II, are assumed to make a major contribution to the first excited state. Structure II is clearly unstable due to the carbocationic centre. In the case of aminoanthraquinones 52c and 52d, donation of the lone pair from the
|
Figure 4.3 Some relevant resonance forms for anthraquinones 52, 52c, 52d and 52f |
amino nitrogen atom markedly stabilises the first excited states, represented respectively by structures III and IV, lowering their energy and leading, as a consequence of the inverse relationship between the difference in energy and the absorption wavelength, to a pronounced bathochromic shift. Two reasons may be proposed for the enhanced bathochromicity of the 1-isomer (52c), compared with the 2-isomer (52d). Firstly the proximity of positive and negative charges in space in the first excited state structure III gives rise to a degree of electrostatic stabilisation. Secondly, the intramolecular hydrogen bonding in structure III, which is not present in structure IV, serves to increase the electronreleasing power of the lone-pair on nitrogen atom and to increase the electron-withdrawing effect of the carbonyl group, both of which effects stabilise the excited state relative to the ground state and lead to a more pronounced bathochromic effect. The 1,4-diamino compound 52f is particularly bathochromic because of an extensively resonance-stabilised first excited state, involving structures V and VI.
 17 сентября, 2015
17 сентября, 2015  Pokraskin
Pokraskin 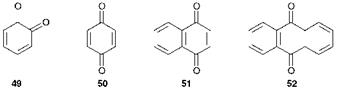
 Опубликовано в рубрике
Опубликовано в рубрике 