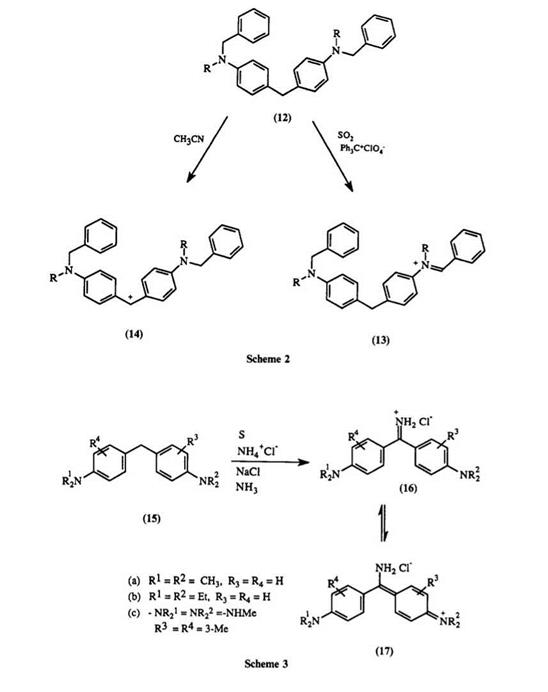
Direct oxidation of diphenylmethanes is of little practical value as color formers. In liquid sulfur dioxide, leuco diphenylmethane 12 (Scheme 2) undergoes hydride abstraction by triphenylcarbenium perchlorate at the benzylic amine position to form immonium ion7 13, whereas in acetonitrile
|
(11) Scheme 1 |
diphenylmethane dye 14 is formed. This example demonstrates the influence that the solvent can have directing effect on the hydride abstraction.
The practical route for oxidizing leuco diphenylmethanes 15 demands inital conversion to an imine salt 16. The imine salt is obtained by heating a mixture of diphenylmethane, sulfur, ammonium chloride, and sodium chloride at 175°C in a current of ammonia; or by heating a mixture of diphenylmethane, urea, sulfamic acid, sulfur, and ammonia at 175°C (Scheme 3). Dyes 16 can be represented as the quinonoid resonance structure 17. Dyes of this class, known as auramines, are all yellow, with the only commercial representative being auramine O 16a. Due to its poor lightfastness and instability to hot acids and bases, its use has been restricted to dyeing and printing cotton, paper, silk, leather, and jute.
The chemical oxidants for triphenylmethanes are categorized into two groups: the strong oxidant group consists of PbO2, Na2Cr2O7/H+, and MnO2, while the mild oxidant group consists of oxygen or air, hydrogen peroxide, nitrobenzene, peroxomonosulfuric acid or its salts. Care must be taken in selecting the proper oxidant and the reaction conditions in order to prevent overoxidation. For example, oxidation of leuco Malachite Green with excess PbO2 leads to decomposition products to quinoneimine and benzoic acid.
|
|
Although PbO2 in HCl has been commonly used, an ecologically safer oxidant is MnO2 in the presence of H3PO48-11 or HCl.9,12 In general, oxidation utilizing oxygen13 or hydrogen peroxide14 requires a catalyst, usually a metal complex. A catalytic amount of halo — or cyano-substituted benzoquinones,15,16 or nitro-substituted phenanthrenequinonen. n has been used in conjunction with metal catalysts. The metal catalysts can be Mn, Fe, Cu, or Cr complexes of porpharines, tetraazac[14]annulene, phthalocyanine, or tetraazacyclodecane14,18 or molybdic acid, and VO+ . The oxidation reaction is carried out in a solvent such as glacial acetic acid.18 For example, leuco Malachite Green in glacial acetic acid has been oxidized with Chloranil14 and air in the presence of an iron tet — raaza[14]annulene complex at 50°C.
The kinetics for the oxidation of leuco bases using oxygen has been studied.19 The oxidation involves complex formation between the proto — nated leuco base and the peroxy radical formed by air oxidation of the solvent. Addition of a radical initiator (AIBN) facilitates the reaction, while radical inhibitors retard the dye formation. In addition, oxidation reactions employing 2,3-dichloro-5,6-dicyanoquinone have shown large isotope effects
in acetonitrile.20
Anodic oxidation has been employed for water-soluble triphenyl- methane dyes. It has been shown that the formation of dye is an irreversible two-electron oxidation process.21-23 This method has been used for the oxidation of diamino triphenylmethane leuco compounds containing two to four sulfonic acid groups to obtain food-grade colored materials.24
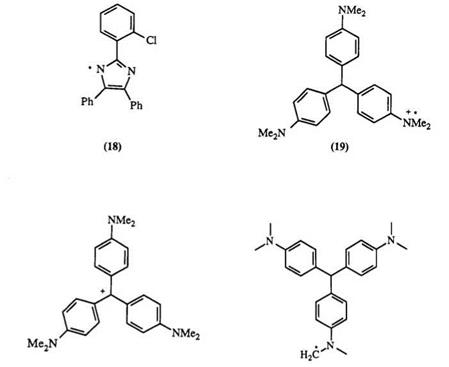
The photochemical oxidation of triphenylmethanes has been studied.25-28 In general, triarylmethanes are photooxidized to dyes under UV irradiation in the presence of hexaarylbisimidazo1es.29-32 The mechanism of the bisimidazole-sensitized photooxidation of leuco Crystal Violet has been studied.33 The first step of the reaction involves an electron- exchange reaction between 2-(o-chiorophenyl)-4,5-diphenylimidazolyl radical 18 (generated from the corresponding dimer) and the leuco dye, forming a solvated electron and a radical cation26,34’35 19. Decay of the radical cation results in the formation of a dye cation 20 and a hydrogen atom. The presence of the radical cation 19, the dye cation 20 as well as radical 21 has been shown by conventional and laser flash photolysis and by spin trap36 experiments. Photolysis of leuco nitriles of Malachite Green and Crystal Violet suggests charge transfer between the electron-donor (-NMe2) and electron-acceptor (-CN) groups.37
When a benzene solution of benzaldehyde and leuco Crystal Violet is irradiated in air, it undergoes an exceptionally fast photochemical reaction and the dye is formed in an excellent yield.38
|
|
 11 августа, 2015
11 августа, 2015  Malyar
Malyar 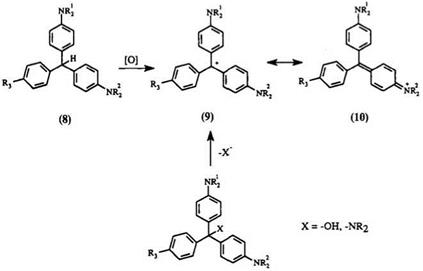
 Опубликовано в рубрике
Опубликовано в рубрике 