 |
The benzhydrols are obtained by reacting an aromatic aldehyde with an arylamine or by oxidation70 of a diphenylmethane derivative with PbO2 or MnO2. The benzhydrol intermediate is then treated with an arene nucleophile in the presence of acid catalysts such as aluminum chloride,71 concentrated hydrochloric acid,55 phosphoric acid,72 or sulfuric acid73 to give triphenylmethane leuco dyes.74,75 Under these conditions, the benzhyd — rol is protonated at the hydroxy group on the central carbon atom. A carbo — cation is formed by the loss of water (Eq. 6). Electrophilic substitution then occurs on the meso carbon atom yielding the desired product. Amino,75
 |
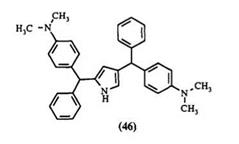 |
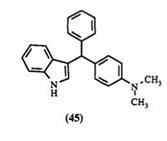
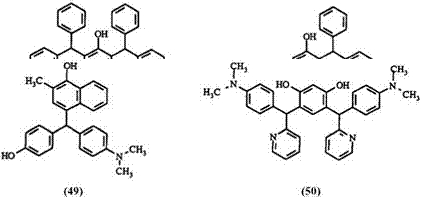
ureido, or bisureido groups15,73,76 have also served as leaving groups. The compounds 45-50 are prepared by this method. The aromatic nucleophiles
 |
usually contain electron-donating groups, but comparatively deactivated aromatic nucleophiles such as sulfonated arylamines and naphthalenes can also be used. For example, 4,4′-bis(dimethylamino)benzhydrol (Michler’s hydrol) reacts with P-naphthyl-3,6-disulfonic acids to give leuco compound
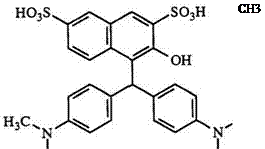 |
51.
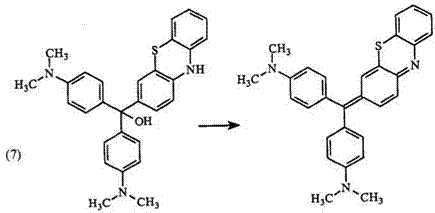 |
Carbinol bases are obtained by treating cationic triphenylmethine dyes with alkali.77 However, not all carbinol bases are stable. For example, 52 readily eliminates water to form the neutral quinonimide45 53 (Eq. 7).
 |
|
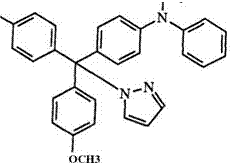
Hydroxy leuco bases can be converted into the corresponding amino leuco bases by allowing the leuco compound to react with a secondary amine in the presence of acetic acid.78 Examples, 54, of amine bases utilized in this manner are imidazole, 1,2,4-triazine, aryl amine, and cyclohexayl amine.
Experimental Procedure19 for 3-[a-(4-dimethylaminophenyl)benzyl]in — dole (45). To a solution of 4-N, N-dimethylaminobenzhydrol (0.45 g, 2 mmol) in MeOH (25 ml) under reflux, was added a solution of indole and concentrated hydrochloric acid (0.8 ml) in water (25 ml). The mixture was heated under reflux for 90 min, followed by the addition of KOH solution (5%, 30ml). After the reaction was allowed to cool, the product was isolated by filtration (0.63 g, 91%).
 13 августа, 2015
13 августа, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 