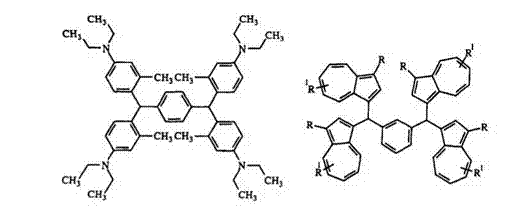
The reaction of an aromatic aldehyde with an aromatic nucleophile containing hydroxy or amino groups gives products with at least two identical phenyl groups19 — 83 e. g., 55, 56. Use of two different nucleophiles generally results in lower yield.
|
|
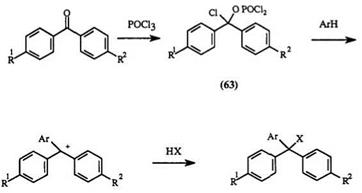
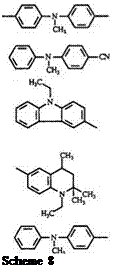
Benzene and naphthalene compounds can be formylated under Vil — smeir conditions. The formyl compounds, with or without isolating, can be condensed with amino arenes to give leuco compounds. In this reaction, the benzhydrol intermediate is not isolated.21,19,84 — 86 The reaction is generally carried out in an alcohol solvent such as isopropanol, butanol, or pentanol and an acid catalyst such as hydrochloric acid, sulfuric acid, or methanesul — fonic acid.81 Acetic acid can also be used both as catalyst and as the solvent. Urea sometimes is added as catalyst.84,88 Terephthaldehyde reacts with N, N-diethyl-3-methylaniline89 and substituted azulenes to give a bis — triphenylmethane21 57 and 58, respectively.
Experimental Procedure19 for 4-bis(4-dimethylaminophenyl)methyl-2,6 dimethoxyphenol (55a). A mixture of syringaldehyde (5.46 g, 30 mmol, N, N-dimethylaniline (1.26 g, 60 mmol), urea (2.1 g), and concentrated sulfur ic acid (4.41 g) in isopropanol (100ml) was heated at 90°C under a nitrogen atmosphere for 24h. The reaction was cooled to room temperature and 40ml of water added followed by 50% NaOH until alkaline. The mixture was filtered and the residue washed with 200ml of cold water. The solid was recrystallized from ethanol and yielded 12.0g (91%), mp 136 -138°C.
Two different nucleophiles can be used but lower yields of the triaryl methane leuco dyes containing three different rings are obtained. For
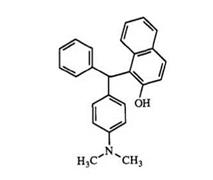
example, a mixture of benzaldehyde, 2-naphthol, and N, N-dimethylaniline gives only 24% yield of the triarylmethane 59. Water-soluble triaryl methanes are also prepared from 4-cyanobenzaldehyde and tetrahydroquino — lines.90
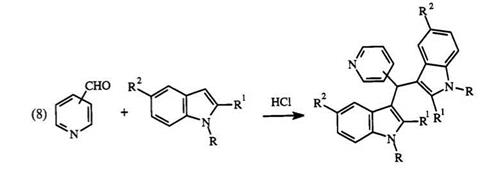
In place of aromatic aldehyde, a hetaryl aldehyde such as 2-pyridinecar — boxaldehyde can also be used.84 If a hetaryl aldehyde such as 4-pyridinecar — boxaldehyde and a hetaryl nucleophile such as indole 61 are used, a trihetarylmethane of type 62 is formed91 (Eq. 8). The reaction normally occurs at the 3-position of indole. However, when the 3-position is substituted, the reaction occurs at the 2-position. Use of two different indoles
|
|
|
|
|
|

 |
 |
|||
R1 R2 Ar
affords the unsymmetrical analogues in low yields, as expected. This product is usually accompanied by the formation of the two symmetrical analogues.
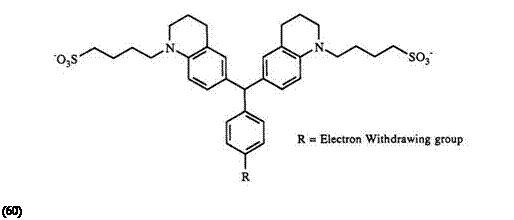
Unlike triarylmethane dyes, comparatively little work has been done with diaryl heteryl-, and aryl diheteryl methane compounds. Analogous to triarylmethanes, triheterylmethane dyes are also prepared using POCl3 and a ketone. The intermediate leuco compounds (similar to 65, see Scheme 8) are not isolated40 in the case of triheterylmethane leuco dyes.
Experimental Procedure91 for 3,3 ‘-diindolyl-4-pyridylmethane (62, R1 = R2 = R3 = H). Indole (2.34g, 0.02mol) and 4-pyridinecarboxal — dehyde (1.07 g, 0.01 mol) are dissolved in ethanol (100 ml). Concentrated HCl (20ml) was added and the mixture allowed to stand at 25°C for 2h. Water (300ml) was added giving a fine white precipitate. The milky suspension was neutralized with concentrated NH4OH, yielding a light yellow precipitate. After filtration and drying, 62 was obtained (2.87 g, 89%), mp 156-158°C.
 16 августа, 2015
16 августа, 2015  Malyar
Malyar 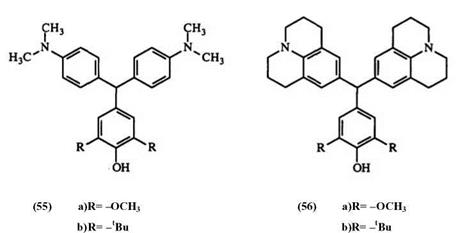
 Опубликовано в рубрике
Опубликовано в рубрике 