There have been very few systematic studies of the electronic spectra of tetrazolium salts. Generally, they have strong UV absorption maxima
|
|
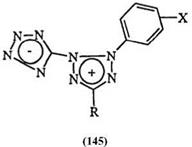
at 240 — 280nm.224 The introduction of electron-donating substituents on the phenyl group at the 2-position leads to a red-shift which extends further on the introduction of an electron-withdrawing substituent in the phenyl group at the 3-position. For example, in 142, R’ = N(CH3)2, the absorption maximum shifts from 440 nm when R is H to 475 nm when R is NO2.82,225 In mesoionic tetrazoliums, e. g., 117, the major absorption band appears at 326—333nm.225 In more conjugated systems such as the dicyanomethylene (143) or the heptafulvalene analogue (144), this band appears at 396 nm in acetonitrile.226 In an interesting extension of this phenomenon, Shchipanov228 prepared a series of mesoionic bis tetrazolium betaines (145). The effect of substituents on the major absorption band is summarized in Table 11.
Table 11. UV/Visible Spectra of Tetrazolium Ylide (145)
|
|
|
R |
X |
X(nm) |
|
CH3 |
H |
275 |
|
C3H7 |
H |
281 |
|
C6H5 |
H |
245 |
|
Styryl |
H |
290 |
|
3-NO2-C6H4 |
H |
235 |
|
4-NO2-C6H4 |
H |
277 |
|
2-HO-C6H4 |
H |
247, 305 |
|
3,4-(OCH3)2C6H3 |
H |
225, 215, 296 |
|
4-(CH3N)2C6H4 |
H |
227, 332, 422 |
|
3-CH3 O-4-HOC6 H3 |
H |
225, 275, 300 |
|
4-NO2 — C6H4 |
4-N(CH3)2 |
275,470 |
|
3-NO3-C6H4 |
4-N(CH3)2 |
230, 257,475 |
|
4-N(CH3)2-C6H4 |
4-N(CH3)2 |
265, 327, 450 |
|
2-HOC6H4 |
4-N(CH3)2 |
255, 300, 452 |
|
CH3 |
4-N(CH,)? |
265, 450 |
|
Ph |
3-NHCOCH3 |
250 |
|
Ph |
3-NH2 |
255, 400 |
|
Ph |
4-N(CH3)2 |
245, 460 |
The spectra of these highly conjugated betaines are solvent sensitive. The absorption maximum of 144 is 450 nm in carbon tetrachloride and 392nm in dimethylsulfoxide. The spectra of a number of substituted diaryl thiobetaines correlated well with Kossower’s Z solvent polarity indicators.227
7.4.2.1. Action of Acids
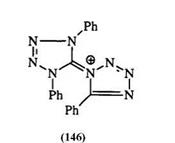
In general, tetrazolium salts are stable in mineral acids. This stability has allowed a number of synthetic transformations such as ester and amide hydrolysis and demethylation of ethers etc.209-212 This is not the case with tetrazolium salts containing a heterocyclic substituent in the 3-position. They tend to decompose in mineral acid to the corresponding tetrazoles.230 Tetrazolyl-substituted tetrazoliums (146) and mesoionic tetrazoles, e. g., 145, are unaffected by acids.228,232
|
|
 24 сентября, 2015
24 сентября, 2015  Malyar
Malyar 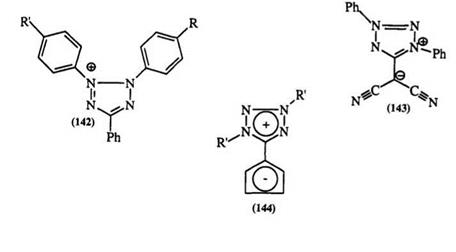
 Опубликовано в рубрике
Опубликовано в рубрике 