RAMAIAH MUTHYALA and XIANGFU LAN
Di — or triarylmethane leuco dyes are those with electron-donating groups such as amino, or hydroxyl substituted at the para or less frequently at the ortho position of phenyl rings. To be of value as dye precursors, at least two amino groups or a combination of hydroxyl and amino groups are required. The amino groups can be primary, secondary, or tertiary. Additional substituents such as carboxylic acids, sulfonic acids, or halogens can also be present. The number, nature, and position of these substituents determine the hue or color of the dye and the type of application. For example, introduction of sulfonic acid group converts the basic dyes into acid dyes; a carboxylic group ortho to the phenolic hydroxyl group converts basic dyes into mordant dyes.
Arylmethane leuco dyes are converted into di — or triarylmethane dyes on oxidation. This class of dye precursors sometimes is referred to as leuco di — or triphenylmethane dyes, or di — or triphenylmethane leuco dyes. The use of the term di — or triarylmethane dyes can be misleading as the central carbon atom is a carbonium ion. Instead, the term di — and triarylmethine dye is recommended for this class as it correlates with the well-known polymethine dyes. Nevertheless, it has not been commonly used.
RAMAIAH MUTHYALA • 3M Company, St. Paul, Minnesota 55144. XIANGFU LAN l Clariant Corporation, Charlotte, North Carolina 28269.
Chemistry and Applications ofLeuco Dyes, edited by Muthyala. Plenum Press, New York, 1997.
Triphenylmethane dyes show inferior lightfastness properties. They are, however, still one of the most important groups of synthetic dyes due to their brilliance, high tinctorial strength, and low cost. Several reviews have appeared on di — and triphenylmethane dyes.1-5 However, the color-forming precursors — leuco dyes — have received less attention in the literature.
In general, the triarylmethane leuco skeleton can be represented by structures 1-4. Traditional leuco di — and triphenylmethane dyes frequently include compounds of type 1 and 3. The closely related compounds 2 and 4 are derived from 1 and 3. Another closely related type is the lactone or phthalide 5 (see Chapter 4). In all of these leuco dyes, one or more of the phenyl rings can be replaced by a hetaryl ring or by a fused aromatic ring such as a naphthalene.
|
(1) X=H (3) X = H (5) (2) X = — NR2,-SO2R (4) X=-OH,-OR,-NR2,-CN, N-Heterocycle,-P(O)OR2 |
R1,R2,R3= — nh2,nr2,-oh
While the classical leuco dyes 1 or 3 form colors by hydride abstraction or oxidation, the leuco dyes 4 or 5 give colored substances on contact with an acid.
Triphenylmethane leuco dyes are far more important than the diphenylmethanes in terms of practical value. Use of triphenylmethane dyes for traditional applications of dyes is limited to dyeing wool, silk, leather, and polyacrylonitrile fibers. The largest portion of the annual production of this class of leuco dyes is consumed in the manufacturing of various copying papers.
The leuco triphenylmethanes are generally stable substances. However, IR studies have shown that in the solid state, some leuco dyes such as 6a-c
|
|
|
|
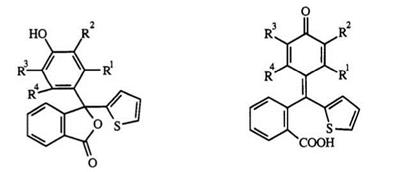
(a) ![]() R1=OH, R2-R4=H
R1=OH, R2-R4=H
(b) R1 = R4 = OH, R2 = R4 = H (e) R1 = R2 = H, R3 = R4 = OH
(c) R1= R2 = OH, R3 = R4 = H (f) R1= R3 = OH, R2 = R4 = H
(g) R1 = R2 = R4 = H, R3 = OH
exist as a mixture of phenol and the quinone carboxylic acids 7a-c, whereas 6d-g exist exclusively as lactone.6
 8 августа, 2015
8 августа, 2015  Malyar
Malyar 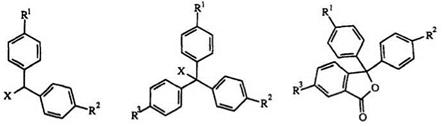
 Опубликовано в рубрике
Опубликовано в рубрике 