Anthraquinone leuco dyes are widely known as vat dyes.10 Vat dyes possess extensively conjugated aromatic systems containing two or more carbonyl groups, e. g., anthraquinone, indigoid chromophores. The colored form of vat dyes are insoluble in water. The dyes are applied by a process whereby the dye is converted to the reduced form (leuco dye) which is soluble in water and can penetrate into a cellulosic fiber. On exposure to the atmosphere the leuco form is oxidized to the original quinoid form which then precipitates as an aggregate. Vat dyes generally have excellent chemical and photochemical stability.
Leuco quinones can be synthesized by two methods, reductive trapping and oxidative coupling. A variety of reagents, e. g., SnCl2, Fe, dithionite, can be used for the reduction. The most general method has been reduction of quinone with reducing agents such as sodium dithionite in alkaline conditions under nitrogen atmosphere. Alternately, reduction of quinones with tin(II) chloride in aqueous hydrochloric acid gives leuco quinones. However, these leuco hydroxy compounds are unstable in air and their isolation requires protection of phenolic groups. Acylation is commonly used for the stabilization. The leuco naphthoquinone 19a is unstable and is rapidly reoxidized to the quinone in air, but the benzoyl ester 19b is quite stable and may be isolated and stored.11 Leuco quinoneimine dyes 20 can be synthesized by oxidative coupling of phenols with arylamines in the presence of moderate oxidizing agents such as potassium ferricyanide in alkaline condi — tions.12’13
|
(19) a, X = H b, X = COPh |
The leuco quinoneimine dyes are unstable to isolate but the substitution of an electron-withdrawing group such as acyl,13 carbamoy1,13 carboxy,13 or arylsulfonyl 12 group at the amino nitrogen atom stabilizes the
|
|
||
|
|||
Scheme 4
leuco form which then can be isolated in a stable state. The general syntheses of the leuco indophenol dyes are shown in Scheme 3.
 |
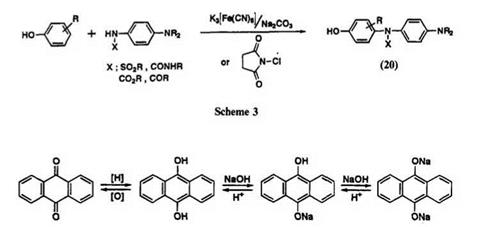
Redox behavior of anthraquinone is shown in Scheme 4. The quinone moiety may be reduced to the hydroquinone form and converted to a leuco salt under alkali conditions. In general, the leuco salt has a strong affinity for cellulose and is soluble in water. The hydroquinone form is insoluble in water and has low affinity to cellulose. The preferred dyeing procedure depends on the structure and properties of the vat dye. The variables that are used to control the process include, e. g., strength and amount of alkali, reduction temperature, and the presence of salts. During the process of reduction, some side reactions, such as overreduction, hydrolysis,
|
(21) |
||
|
Indenthorne |
Blue leuco salt |
Brown leuco salt (over reduction) |
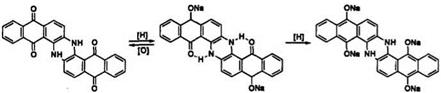
saponification of amido groups, dehalogenation, and keto-enol isomerism, are possible. Premature crystallization of leuco salt is undesirable during dyeing. These side reactions should be avoided to achieve good dyeing performance of vat dyes. Overreduction of indanthrone (21) produces brown leuco salt which has poor affinity compared with that of blue leuco salt (Scheme 5).
 9 июля, 2015
9 июля, 2015  Malyar
Malyar 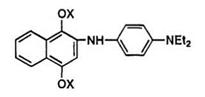
 Опубликовано в рубрике
Опубликовано в рубрике 