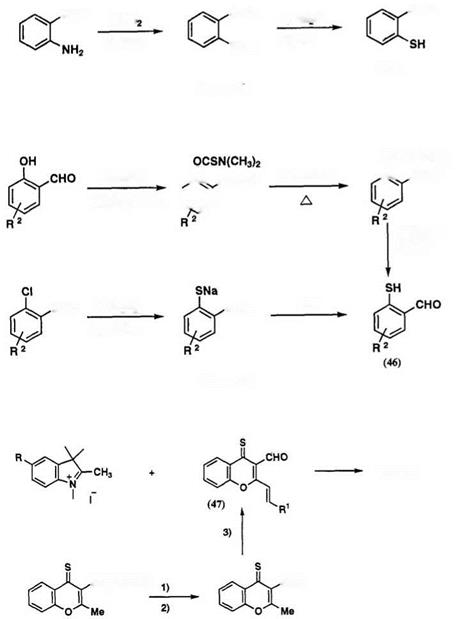
Spiroindolinobenzothiopyrans can be prepared by condensation of Fischer’s base with thiosalicylaldehyde derivatives 46 in ethanol, as shown in Scheme 22.71,89’93 Reaction of 1,2,3,3-tetramethylindolinium salt with carbamoylthiobenzaldehyde,92 which is an intermediate for preparation of thiosalicylaldehyde, also gives the spirobenzothiopyran in high yield via the corresponding indolinium salt, as shown in Scheme 22.94 Conversion of spirobenzopyrans to the corresponding spirobenzothiopyran by phosphorous pentasulfide in pyridine or xylene is possible, but the purification of the product is difficult.
Although thiosalicylaldehyde 46a (R[1] = H) was first synthesized by Friedlander and Lenk (Scheme 23),95 it is an unstable intermediate and should be stored in solution below 0°C. Alternate synthetic procedures utilizing o-chlorobenzaldehyde or salicylaldehyde, as starting materials, are shown in Scheme 24.96 The preferred method for the synthesis of substituted thiosalicylaldehydes 46 is via salicylaldehyde.
|
DABCO:1,4 — diazabicyclo[2.2.2]octane |
|
|
||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||

CHO 1) Na2S2 2
CHO HCI
|
![]() CH2OCOCH3
CH2OCOCH3
1) 5% HCI, MeOH, at 60°C 2)pyridinium dichromate, CH2C12 (48) 3) Me2NCH(OMe)2, benzene, reflux
Spirothiopyrans 45b including a benzopyrylium ring have been prepared in one step by condensation of 2-aminovinyl-3-formylchromone-4- thione 47 with 1,2,3,3-tetramethylindolinium salts in ethanol (Scheme 25).90 The precursor 47 is prepared from 3-carboxymethylene-2-methyl-chromone — 4-thione 48. First, oxidation of 48 with pyridinium dichromate in CH2C12, and then condensation with dimethyl formamide dimethyl acetal in benzene gave compound 47.
Experimental Preparation of 5′,6-dinitrospirobenzothiopyran 44b. A mixture of 5-nitro-1,3,3-trimethyl-2-methyleneindoline (0.38 g, 1.75 mmol) and 5-nitro-thiosalicylaldehyde (0.22 g, 1.2 mmol) in EtOH (50 ml) was refluxed for 2h, and then evaporated under reduced pressure. The residue was chromatographed on silica gel with benzene-acetone (15:1, v/v) and recrystallized from dichloromethane-hexane to give 44b (R1 = NO2, R2 = 6-NO2)(0.30 g, yield 65%).
Preparation of Spirothiopyranobenzopyrylium iodide 45b. A mixture of 2,3,3,4-tetramethylindolinium iodide (0.090 g, 0.3 mmol) and 2-(N, N-di — methylaminovinyl)-3-formyl-chromone-4-thione 47 [R1 = N(Me)2] (0.086 g, 0.33 mmol) in EtOH (10 ml) was refluxed for 1 h. After cooling, the reaction mixture was poured into 100 ml of ether. The precipitate was filtered off and dispersed in 50 ml of ether with stirring for 15 min, followed by filtration. Repeating this procedure gave a pure spirothiopyranobenzopyrylium iodide 45b (0.13 g, yield 80%).
 3 июля, 2015
3 июля, 2015  Malyar
Malyar 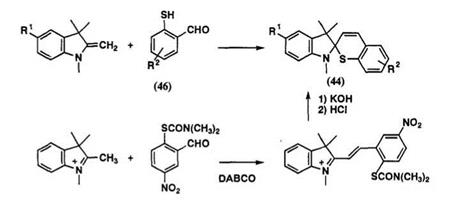
 Опубликовано в рубрике
Опубликовано в рубрике 