Spironaphthooxazines are generally prepared by condensation of 2- alkylidene heterocyclic compounds with an o-nitrosonaphthol in methanol or ethanol, as shown in Scheme 18.73
o-Nitrosonaphthol is prepared by reaction of P-naphthol with sodium nitrite in aqueous solution.74 Similarly, 5-nitroso-6-quinoline, 9-nitroso-10- phenanthol, and other o-nitroso arylols useful for the preparation of spirooxazine derivatives, have been prepared.72 Only one absorption band (Vmax 500 nm) for the colored form of 1,3,3-trimethylspiroindolinobenzo-
|
|
|
Table 5. Xmax of the Colored Form of the Spirooxazine Series in Toluene
|
|
(33)590nm73,77,78 (34) 590nm78
|
|
|
|
|
xazine 38 was listed in the previous review,1 and other spectral data for this class are very limited. Other spiroindolinobenzoxazines from 3- or 5- methoxy-2-nitrosophenol or 3,3 ‘-dinitroso-4,4 ‘-dihydroxy diphenylmethane have been documented in the patents.75,76
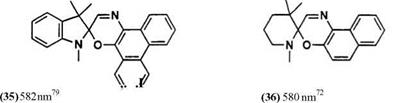
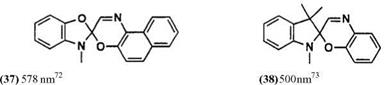
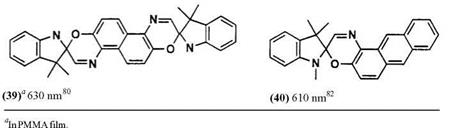
Indolines, benzoxazole, and benzothiazole are possible as 2-methylene heterocycles. The number of known spirooxazine derivatives is much less than for the spiropyrans. This may be partly due to lack of many substituted o-nitrosonaphthols and partly due to lack of sufficient stability of spiro — oxazines. The structures of parent spirooxazines and the Xmax of their photomerocyanine forms are listed in Table 5. The Xmax of the colored forms of compounds 41-43 are not described in the literature.
Experimental Preparation of Spironaphthooxazine 33 (N-Bu). Tri-
ethylamine (3.54 g, 35 mmol) was added to a suspension of 2,3,3-trimethyl — N-butylindolinium iodide (12.0 g, 35 mmol) and o-nitrosonaphthol (6.1 g, 35 mmol) in EtOH (100 ml) under stirring. The mixture was refluxed for 2 h, cooled, and evaporated under reduced pressure. The residue was chromatographed on silica gel with benzene as an eluent, and then recrystallized from methanol to give spiro(N-butylindolinonaphthooxazine) 33 (6.6 g, yield 51%).
 26 июня, 2015
26 июня, 2015  Malyar
Malyar 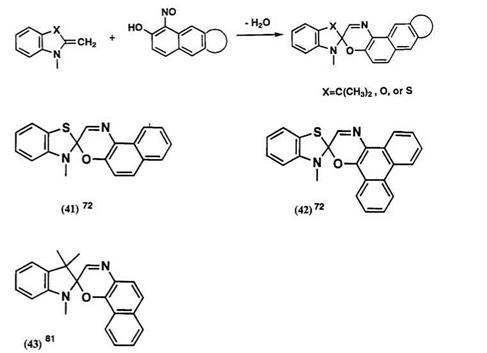

 Опубликовано в рубрике
Опубликовано в рубрике 