In 1963, Bloom and Hutton suggested5 the structure of leuco quinizarin in solution as 9,10-dihydroxy-2,3-dihydro-1,4-anthraquinone (9a). In 1981, Kikuchi and colleagues6 confirmed the structure by means of 1H — and
|
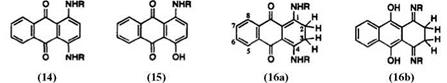
|
13C-NMR. The structures of the leuco derivatives of 1,4-bis(butylamino)- anthraquinone (14) and 1-butylamino-4-hydroxyanthraquinone (15) have been shown to be 1,4-bis(butylamino)-2,3-dihydroanthracene-9,10-dione (16a) and 1-butylamino-10-hydroxy-2,3-dihydroanthracene-4,9-dione (17a), respectively. On the other hand, leuco-1,4-dimethoxyanthraquinone has been assigned the structure, 1,4-dimethoxy-9,10-dihydroxyanthracene (18).
In proton NMR, 1,4-disubstituted anthraquinones show aromatic protons (5,8- and 6,7-positions) as A2B2 type, and the other aromatic proton signals (2,3-positions) as A2 or AB type. Proton NMR data of leuco-1,4- disubstituted anthraquinones in deuteriochloroform are given in Table 1. Leuco-1,4-dimethoxyanthraquinone (18) has hydroxy protons (2H) exhibiting a singlet, at 9.78 ppm. As expected, aromatic protons appear as A2B2 and A2 type. On the other hand, leuco-1,4-bis(butylamino)anthraquinones (16, R = и-Bu) do not show an A2-type peak for 2,3-aromatic protons but rather a sharp singlet at 2.70 ppm. The j9-quinoid structure for 16a has been assigned based on 13C chemical shift of the carbonyl group, observed at 172.2 ppm (Table 2).
|
|
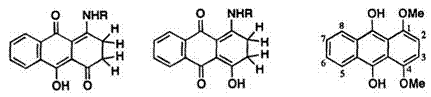 |
In the proton NMR spectrum of leuco- 1-hydroxy-4-butylaminoanthra — quinone (17) a triplet at 2.88ppm due to the methylene protons (4H), a sharp singlet at 13.95ppm corresponding to one hydroxy proton, and a broad signal at 14.90ppm from one amino proton have been observed. These results permit the structural assignment of 17 to 17a and 17b.
Two structures 9a or 9b are possible for leuco quinizarin (9). Bloom and Hutton5 have proposed the structure of 9 to be 9a by comparing the chemical shift of methylene protons with those of leuco naphthazarin (3.05 ppm) and leuco naphthoquinone (3.08 ppm). However, based on UV spectra and chemical reactivity, Egerton and CO-workers7 and Greenhalgh8 independently suggested an equilibrium mixture of 9a and 9b in solution.
The 13C NMR spectral data for 9, 16, and 17 are shown in Table 2. The chemical shifts of carbonyl carbons of anthraquinones are characteristically observed at about 180 ppm.9 The chemical shifts for carbonyl carbons of 1,4-naphthoquinone and 1,4-benzoquinone appear at about 185 ppm, while those of carbonyl carbons adjacent to a methylene or methyl carbon are at about 200 ppm. The chemical shifts of the C1 and C4 of 9 are observed at 200.8 ppm and assigned to the 1,4-diketo form 9a. In the 13C spectrum of 17, the chemical shifts of carbonyl carbons are at 199.9 and 172.2 ppm. The former value corresponds to a carbonyl carbon adjacent to the methylene carbon, and the latter corresponds to the carbonyl carbon in the 9-position. The methylene carbons of 17 show two signals at 34.5 and 23.8ppm. From these results, 17 is considered to exist exclusively as an unsymmetrical 4,9-diketo form, 17a. Thus, these NMR spectral data suggest
|
Table 1. 1H-NMR Chemical Shifts of Leuco Anthraquinones6
|
|
|
Compound’1 |
Cl |
C2 |
C3 |
C4 |
C5 |
C6 |
C7 |
C8 |
C9 |
CIO |
Cll |
C12 |
C13 |
C14 |
|
9 |
200.8 |
35.7 |
35.7 |
200.8 |
124.4 |
130.4 |
130.4 |
124.4 |
154.9 |
154.9 |
107.3 |
107.3 |
129.1 |
129.1 |
|
16(R = »-Bu)z’ |
162.1 |
22.9 |
22.9 |
162.1 |
125.7 |
129.9 |
129.9 |
125.7 |
172.2 |
172.2 |
102.2 |
102.2 |
135.7 |
135.7 |
|
17 (R=»-Bu)b |
165.1 |
23.8 |
34.5 |
199.9 |
124.4 |
130.1 |
129.6 |
125.7 |
153.0 |
172.2 |
107.3 |
101.8 |
129.9 |
135.2 |
|
Table 2. 13C-NMR Chemical Shifts of Leuco Anthraquinones6 |
|
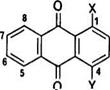
^Numbering system of anthraquinone ring as follows: |
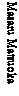 ^The n-butyl group was observed as follows: (16) 13.9, 20.3, 31.9,43.2 ppm; (17) 13.6,20.1,31.6,43.6ppm.
^The n-butyl group was observed as follows: (16) 13.9, 20.3, 31.9,43.2 ppm; (17) 13.6,20.1,31.6,43.6ppm.
that the structures of leuco anthraquinones may differ depending on the substituents, e. g., 9 as 1,4-quinone, 16 as 9,10-quinone, 17 as 4,9-quinone, and 18 as 9,10-dihydroxyanthracene structures.
 9 июля, 2015
9 июля, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 