Formazans can be characterized as tautomeric structures 178 and 179. Schiele proposed that, in some cases at least, formazans are better represented by the delocalized structure 180 implying coplanarity among substituents.301302
1,3,5-Triphenylformazan can be isolated in two forms; the red form is assigned to the tautomeric structure 178 or 179 and the yellow form is assigned to the trans-anti geometry 181.303,304 This assignment is supported by IR, NMR, X-ray diffraction as well as kinetic studies.245,305-315
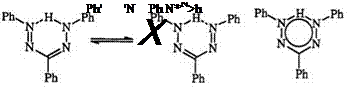 |
Unsymmetrically substituted formazans form a complex mixture of valence tautomers and geometric isomers with different conformations. The tautomerism is further complicated when substituents on the aryl ring are
|
|
|
|
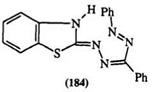
capable of either deprotonation or hydrogen bonding. Thus, in the case of 1-(2-aminopheny1)-3,5-diphenyl formazan (182), using proton and carbon — 13 NMR as well as electronic and IR spectra, Ostrovskaya and colleagues proposed a complex equilibrium mixture of six isomers.316 Equally complex equilibria arise from the introduction of a carboxylic group in the ortho position of the 1-aryl substituent, e. g., 183.317’318 For formazans, containing a heterocyclic ring at the 1-position, e. g., the benzothiazolyl formazan (184), a total of nine geometric isomers are possible.319-324
 1 октября, 2015
1 октября, 2015  Malyar
Malyar 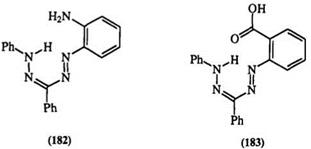
 Опубликовано в рубрике
Опубликовано в рубрике 