1.4.1. Molecular Design for the Near-IR Dyes
Becker and Kolc first examined the photochromism of spiroindo- linobenzothiopyran 44, a thio analogue of spiroindolinobenzopyran (Scheme 19). The closed form of this spirobenzothiopyran is stable and the photocoloration is very slow, compared with spiropyrans.71
In recent years, much effort has been focused on the development of new spirothiopyrans having absorption maxima of the colored form within the range of the oscillation wavelength of semiconductor lasers. The length of п-conjugation from nitrogen atom in the indoline component to sulfur atom of thiolate is fixed to make up the spiro skeleton and additional conjugation cannot be inserted to this main п-conjugation. Substituents that produce bathochromic shifts of the colored form are limited. However, 5′,6-dinitro derivative gives the most bathochromic shift.13,89 Its colored form has the absorption maximum in the near infrared region, and is available for application in erasable optical data storage using a semiconductor laser (780-830 nm).
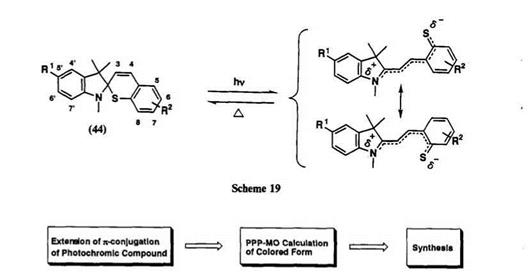
Another approach to shift absorption bands for the colored form is the extension of п-conjugation outside the spiro skeleton. Procedures of molecular designs for such photochromic compounds are shown in Scheme 20. (1) Position for extension of additional п-conjugation in spirothiopyran
|
|
|
r45 and 45’ |
X |
D |
R |
|
a |
O |
H |
H |
|
b |
O |
-CH=CH-NMe2 |
H |
|
c |
O |
_CH=CH-NMe2 |
NO2 |
|
d |
O |
-CH=CH-C6H4-p-NMe2 |
H |
|
e |
O |
-CH=CH-C6H4-p-NMe2 |
NO2 |
|
f |
S |
-CH=CH-C6H4-p-NMe2 |
H |
|
Scheme 21 |
should be considered outside the spirothiopyran skeleton. For example, in compound 45a (Scheme 21), the extension of n-conjugation is possible by introducing electron-donating substituent at positions 4 and 6 to 9 in the thiopyran component. (2) The absorption maxima of various colored forms with extended п-conjugation are calculated by PPP-MO. For example, in 45, absorption maxima of merocyanine dyes with extended п-conjugation at 4-position calculated by PPP-MO are listed in Table 6. Merocyanine dyes 45e and 45f having calculated absorption band above 700nm are selected. (3) The preparation of these spirothiopyrans is planned.
The colored form of spiropyrans 10 presented in Table 3, which shows Vax in the near IR, has been prepared using similar molecular design.13 In contrast to spiropyran 10, the merocyanine form 45" is unstable, and quickly changed to the spiro form 45. The thermal stability is affected by presence
|
Table 6. PPPCalculationofMerocyanineDyes 45b — 45f
aOscillator strength. |
of a nitro group. Compounds 45c and 45e include a nitro group in the indoline component, and no longer exhibit photochromic behavior at room temperature.
With the help of similar molecular design, spirothiopyranonaphtho — pyrans 19 with absorption band in the near IR (692-850nm) on UV irradiation have been prepared.52 As predicted, the photocoloration of these spirothiopyrans occurs, but the reverse reaction to colorless form does not occur in solution as extension of п-conjugation increases.
 2 июля, 2015
2 июля, 2015  Malyar
Malyar 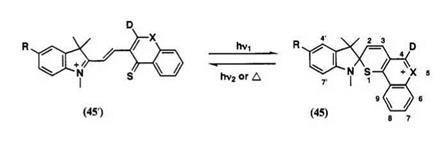
 Опубликовано в рубрике
Опубликовано в рубрике 