Spirooxazine is an aza analogue of spiropyran in which the carbon atom at 3-position is replaced by a nitrogen atom. Historically, the photochromic phenomenon of spiroindolinooxazine derivatives was found after discovery of photochromic spiroindolinobenzopyran.72
Spironaphthooxazine 33 is commercially available as a photochromic compound. Due to its excellent lightfastness, many spironaphthooxazines have been synthesized and their photochromic properties have been investigated for industrial applications. Spironaphthooxazine is colorless (Xmax < 400 nm), and its photomerocyanine form mainly gives blue color.
|
|
Very little is known about the parent benzoxazine analogue,1 due to difficulties in the preparation of o-nitrosophenol. Synthetic procedures and practical application of spironaphthooxazines can be found in the patent literature and have been reviewed.72
The name used by the Chemical Abstracts for spironaphthooxazine 33 is 1,3-dihydrospiro[2#-indole-2,3′-[3#]naphtho[2,1-b][1,4]-oxazine]. The
numbering is shown in Scheme 17.
 26 июня, 2015
26 июня, 2015  Malyar
Malyar 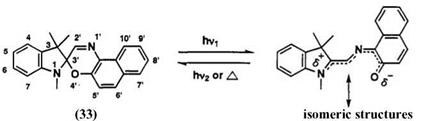
 Опубликовано в рубрике
Опубликовано в рубрике 