It is well known that quinizarin (22) is alkylaminated in air to give a mixture of 1-alkylamino-4-hydroxyanthraquinone (23), 1,4-bis(alkylamino)- anthraquinone (24), and 2-alkylaminoquinizarin (25) (Scheme 7). The reaction conditions affect the ratio of these products. In a nitrogen atmosphere, or in the presence of sodium dithionite as reducing agent, the main amination product is 24. The solvent effects of the reaction of leuco
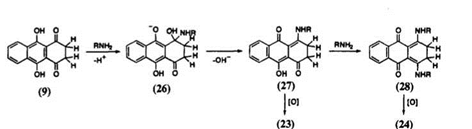
quinizarin (9) with butylamine have been studied in detail by Kikuchi and co-workers6’15 (Scheme 8). The same workers have also calculated thermodynamic parameters of this reaction.
Kikuchi et al. have observed that the initial attack of amine occurs at the carbonyl carbon, resulting in the formation of an ionic intermediate 26. This reaction is very sensitive to the solvent polarity. Under nitrogen atmosphere, intermediate 27 is further aminated to give 28. Oxidation of 27 and 28 gives 23 and 24, respectively. Oxidation in nitrobenzene, however, results in dealkylation products. In the presence of air and triethylamine, decomposition of aminoanthraquinones occurs.
The alkylamination of quinizarin (22) in the presence of copper salts has been studied by Matsuoka and co-workers.16 The reaction proceeds via oxidation by copper ion of 22 to quinizarinoquinone (29). 2-Alkylamino- quinizarin (25) was obtained in quantitative yield. The 1,2-ring-closure product (30) is obtained by the reaction of 22 with ethylenediamines in the presence of copper ions17 (Scheme 9).
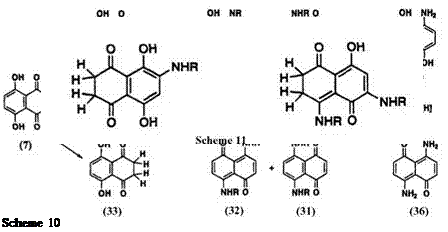
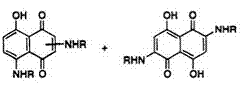
By contrast, alkylamination of naphthazarin (7) in the presence of sodium dithionite followed by oxidation gives 1,4-bis(alkylamino)-5,8-naph — thoquinone (31).18,19 However, Kikuchi and co-workers20 obtained isomeric 1,5-bis(alkylamino)-4,8-naphthoquinone (32) from the reaction of leuco naphthazarin (33) with alkylamine They also isolated 5-alkylamino — leuco-naphthazarin (34) as an intermediate, which is further aminated at the 1-position to give 32. Bloom and Dudek21 have studied the structure of leuco aminonaphthoquinones and their tautomeric equilibria in solution. They concluded that the reaction of leuco naphthazarin (33) or the leuco compound (35) derived from 1,5-diamino-4,8-naphthoquinone (36) with methylamine gives mixtures of 1,4-bis(methylamino)-31 (R = Me) and 1,5- bis(methy1amino)naphthoquinones 32 (R = Me) after oxidation of leuco aminonaphthoquinones (Scheme 10). Some of the structures of leuco aminonaphthoquinones are shown in Scheme 11.20
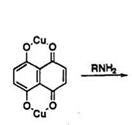
Alkylamination of naphthazarin copper complex (37)22 gives predominantly a mixture of 2(or 3),5-bis(alkylamino)-8-hydroxy-1,4-naphtho- quinone (38) and 2,6-bis(alkylamino)-4,8-dihydroxy-1,5-naphthoquinone
|
|
 |
(39), together with small amounts of 2-alkylamino-, and tris(alkylamino)- naphthazarin (Scheme 12). The structure of 39 has recently been assigned by NOESY NMR.23
 |
||
 |
||
The redox behavior of aminonaphthoquinones has been investigated by Matsuoka and co-workers.11 Reduction of quinoxaline quinone (40) by sodium dithionite in aqueous sodium hydroxide gives the corresponding leuco dye (41) which absorbs at 445nm. Compound 40 shows quinone-
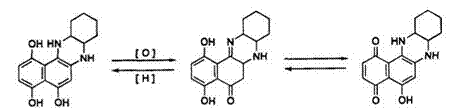
quinoneimine tautomerism in chloroform solution and absorbs over the full range of the visible region. The leuco dye (41) can be isolated in a stable state and can be easily reoxidized to dye 40 by an oxidizing agent such as hydrogen peroxide. Dye 40 was regenerated as a mixture of two tautomers (40a and 40b) (Scheme 13).
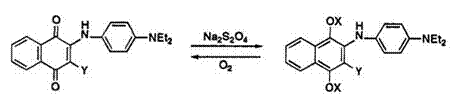
Reduction of 2-arylaminonaphthoquinone dye 42a (^^ 560 nm) with sodium dithionite under a nitrogen atmosphere gives the leuco dye 43a, which has an absorption maximum at 376 nm and is colorless. However, the leuco dye 43a was immediately reoxidized to dye 42a by atmospheric oxygen, although it could be isolated in a stable state as benzoyl ester (43b) (Scheme 14).11
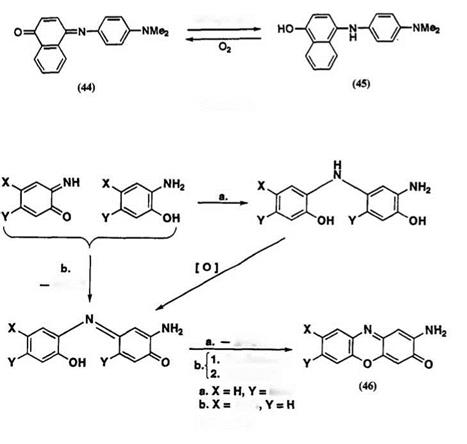
The leuco dye 45 can be obtained by the reduction of indonaphthol dye 44 (^шах 455 and 630 nm) with tin(II) chloride in aqueous hydrochloric acid. The leuco dye 45 has maximum absorption at 300nm and is colorless. Isolation of 45 was unsuccessful owing to the rapid reoxidization of 45 to 44 by atmospheric oxygen (Scheme 15).11 Stabilization of 45 was attained by acylation, etc. (Scheme 3).
Polyhydroxybenzenes or aminophenol derivatives can be considered as leuco quinones or leuco quinoneimines. They are easily oxidized and couple
![]()
|
|
|
|
|
MeOH
Cycllzatlon
Oxidation
MeO
MeO
![]() Scheme
Scheme
in the oxidized form to afford quinoneimine-type heterocyclic dyes such as phenoxazine or phenazine dyes. The autoxidation reaction of these leuco quinoid compounds has been used in hair dyes.24 Autoxidation of 4- or 5-methoxy-2-aminophenols has been found to yield the corresponding 7- or 8-methoxy-2-aminophenoxazin-3-ones (46), which give orange-brown colors (Scheme 16).
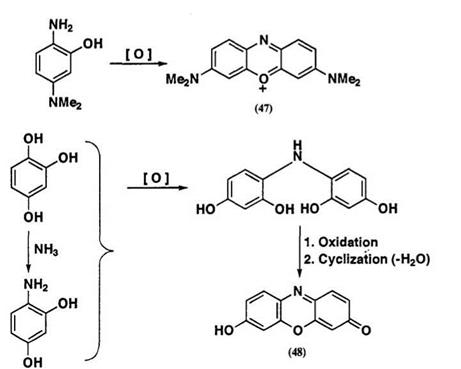
The blue dye (47), formed from the autoxidation of 4-N, N-dimethyl- amino-2-hydroxyaniline, is the oxygen analogue of methylene blue. The autoxidation of 1,2,4-trihydroxybenzene, carried out in the presence of ammonia, gives the hydroxyphenoxazinone dye (48) via a 2,4-dihydroxyani — line intermediate (Scheme 17). Many types of phenoxazines, phenazines, and phenoxazinium salts can be obtained by autoxidation of polyhydroxyben — zenes and their amino derivatives. Some autoxidative dyes may give poly-
|
Scheme 17 |
meric species as a result of carbon-carbon or carbon-nitrogen oxidative coupling.
 12 июля, 2015
12 июля, 2015  Malyar
Malyar 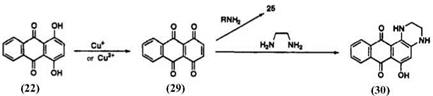
 Опубликовано в рубрике
Опубликовано в рубрике 