The reaction of 2′-aminofluorans with aralkyl halides is solely used to prepare 2′-diaralkylaminofluorans developing red or green colors.
Thus, 2′-aminofluorans (82) reacts with benzyl chloride in organic solvent such as isopropanol or toluene at reflux in the presence of potassium carbonate or sodium carbonate to give 2′-dibenzylaminofluorans (83) in excellent yield. (Eq. 7).
|
|
Besides benzyl chloride, methyl — and/or chlorine-substituted benzyl chlorides, phenethyl chloride, etc. are also successfully employed to give 2′-diaralkylaminofluorans in excellent yield. However, aryl halides such as chlorobenzene and bromobenzene hardly enable the reaction, though aryl iodides such as iodobenzene give 2′-diarylaminofluorans in low yield.
Table 8 shows melting points of 2′-dibenzylaminofluorans (83).
|
|
|
83 |
Ri |
R2 |
R3 |
О 0 % |
|
a |
C2H5 |
C2H5 |
H |
173-174 |
|
b |
C2H5 |
C2H5 |
3′-CH3 |
152-155 |
|
c |
C2H5 |
C2H5 |
3-C2 H5 |
173-175 |
|
d |
C2H5 |
C2H5 |
4′-Cl |
165-166 |
|
e |
C2H5 |
C2H5 |
4′-CH3O |
184-186 |
|
f |
-(CH2E- |
3′-CH3 |
213-215 |
|
|
g |
C2H5 |
4-CH3 C6 H4 |
4′-CH3O |
162-165 |
Preparation of 2′-Dibenzylamino-6′-diethylaminofluoran (83a). A mix
ture of 2′-amino-6′-diethylaminofluoran (0.1 mol), benzyl chloride (0.4 mol), and potassium carbonate (0.2 mol) in isopropanol (100 ml) was stirred under reflux until the reaction was complete: the reaction progress was easily monitored by TLC. Then, isopropanol was distilled out, and toluene (400ml) and water (100ml) were added. After refluxing for 1 h, the toluene layer was separated, washed with hot water, and concentrated. The residue was refluxed with methanol (200ml) for 1 h. After being cooled, the precipitate was filtered off, washed with methanol, and dried to give 2′-dibenzylamino-6′-diethylaminofluoran in 85% yield as a pale green crystalline powder, mp 173- 174 °C.
 3 сентября, 2015
3 сентября, 2015  Malyar
Malyar 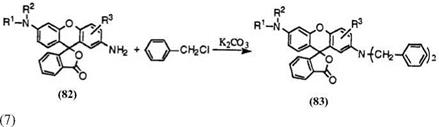
 Опубликовано в рубрике
Опубликовано в рубрике 