6.2.1. Color-Formation Reaction
Colorless or nearly colorless fluoran compounds having appropriate substituent(s) react with acidic compounds to open their lactone rings resulting in extension of the conjugated double bond system, enabling color formation. Lactone ring opening can be determined very easily by the disappearance of lactone absorption around 1760 cm-1 in the infrared spectrum. For example, a solution of fluoran 12 in toluene develops black color on the addition of an acidic compound (Figure 6.1).
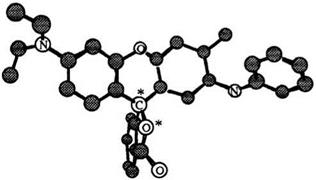
A perspective view of 12 is shown in Figure 6.2. X-ray structure analysis8 on the fluoran shows that the xanthene moiety is slightly bent
|
colorless form colored form Figure 6.1. Color-formation reaction of fluoran 12. |
|
Figure 6.2. Perspective view of fluoran 12. |
along the line of spiro-carbon (C*) and oxygen atoms, and the phthalide moiety is almost perpendicular to the xanthene moiety. The C*—O* length is 1.527 A which is about 0.1 A longer than the usual C(sp3)—O length. This elongation gives easy cleavage of the C*—O* bond to open the lactone ring resulting in colored structure.
The color-formation reaction is not irreversible but reversible. Thus, the colored form can easily reproduce the colorless form by treating with base.
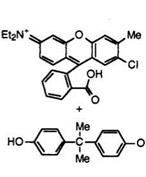
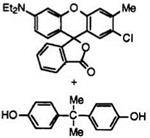
When solid organic compounds such as 4,4′-isopropylidenediphenol (Bisphenol A) are used as the acidic compound, higher alcohols can. control the reversible color-formation reaction. For example, a molten mixture of fluoran 9 (1 part) and Bisphenol A (5 parts) develops vermilion color. In the presence of 1-hexadecanol (94 parts) the resulting mixture indefinitely repeats colorless and colored forms above and below ca.48°C or the melting point of 1-hexadecanol, respectively (Figure 6.3). That is, below the melting point of 1-hexadecanol the affinity of the fluoran compound with Bisphenol A is stronger than that of 1-hexadecanol with Bisphenol A resulting in the color-formation reaction. On the other hand, above the melting point of 1-hexadecanol it functions as an inhibitor of the color-
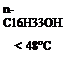 |
 |
 |
colorless colored
Figure 6.3. Reversible color-formation reaction between fluoran 9 and Bisphenol A.
formation reaction. Besides higher alcohols, certain esters, ethers, ketones, nitriles, and other compounds can also control the reversible color-formation reaction. This remarkable property is used as a thermoindicator.9
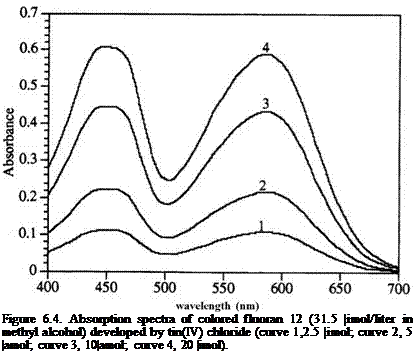 |
Figure 6.4 shows the absorption spectra of the colored form of fluoran 12 developed by tin(IV) chloride in methyl alcohol. It is clear that the
colored form of 12 increases proportionally with increasing amount of tin(IV) chloride and the color formation is complete by ca. 0.5 mol of tin(IV) chloride per mol of 12 to give the absorptivities of ca. 40 liters g-1 cm-1 at 450 and 585 nm.
 23 августа, 2015
23 августа, 2015  Malyar
Malyar 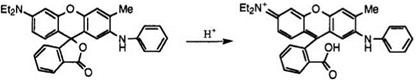
 Опубликовано в рубрике
Опубликовано в рубрике 