Though the colored form of unsubstituted spirobenzothiopyran is unstable, the nitrosubstituent leads to stabilization of the colored form. The absorption maxima of some 6-nitrospiroindolinobenzothiopyrans 44 in a
|
Table 7. Absorption Maxima of the Colored Form of Spiroindolinobenzothiopyran 44 in Vinyl Chloride-Vinylidene Chloride Copolymer
|
polymer film have been measured (Table 7).89 The absorption spectra of the colorless and colored form of 6-nitrospiroindolinobenzothiopyran are shown in Figure 1.9. The colorless form does not have absorption bands in the visible region, and the colored form has a broad band in the near infrared region indicating the absorption band significantly shifts to longer wavelength, compared to spirobenzopyran.93
Substituent effects on the Xmax are remarkable. Electron-withdrawing groups at the 5′-position, e. g., 5 ‘-nitro-substitution (indoline component), and donor substituent at the 8-position (benzothiopyran component) in 44 leads to a longer wavelength shift. As the polarity of the solvent increases, the Xmax of the colored form of spiroindolinobenzothiopyran results in hypsochromic shift. This can be interpreted as the existence of a polar structural component of the colored form in the ground state. Kinetic study has suggested that the zwitterionic structure largely contributes to the colored form of 6-nitrospiroindolinobenzothiopyran, as well as spiropy — rans.97 Based on 1H-NMR and X-ray analysis,98,99 the existence of an
|
Figure 1.9. Absorption spectra of (a) the colorless form and (b) the colored form of 1’3′,3′-trimethyl-6-nitrospiroindolinobenzothiopyran 44a (R1 = H, R2 = 6-NO2) produced by irradiation with UV light in PVC film (1.0 wt%) at 23°C. |
equilibrium mixture of trans-cis and trans-trans isomers for the colored form of 8-methacryloxymethylspiroindolinobenzothiopyran is proposed.
In the spirothiopyran 45b, the spiro form has two absorptions in the visible region (^max 490 and 474nm) due to a polyene chromophore from V-vinyl group to oxygen of the benzopyrylium component.90 The colored form of 45b produced by visible light irradiation shows the ^max at 570 nm. This colored form 45b" was confirmed by characteristic 1H-NMR spectra, as well as that of spiropyran.
Quantum yields of the coloration of spiroindolinobenzothiopyran are very small, compared to those of the spirobenzopyrans. For example, for 6-nitro-8,5-dimethoxyspiroindolinobenzothiopyran the quantum yield is 0.048 in EtOH, and 0.018 in DMF.14 The mechanism of photocoloration of spiroindolinobenzothiopyrans involves the excited singlet state, but not the excited triplet state, as shown from experiments using ferrocene as a triplet quencher. The low quantum yield may be due to deactivating influence of sulfur atom in 44. Nevertheless, 44 follows the same mechanism as that of normal spiropyran. The thermal fading rate constant at 25°C (conversion from the colored form to the spiro form) is 10- 1 to 10-4 s — 1 depending on the solvent. A repeat of 30 cycles of photothermochromism was required to achieve 50% photodegradation in degassed DMF.97
The structures of some spiroindolinobenzothiopyrans have been determined by X-ray crystallography. Selective bond lengths of spirothiopyrans
|
Table 8. Selected Bond Length (A) of Spirothiopyrans 44a (R1 = H, R2 = 6-NO2) and 45b
^Estimated standard deviations for 44a and 45b are 0.01 and 0.02-0.03 A, respectively. ^Numbering for compounds 45b is C4—C12, C11—C12, and S1-C11. |
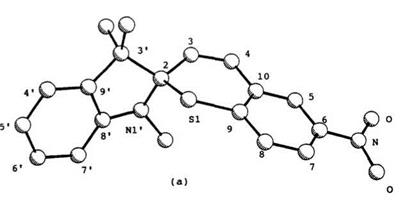
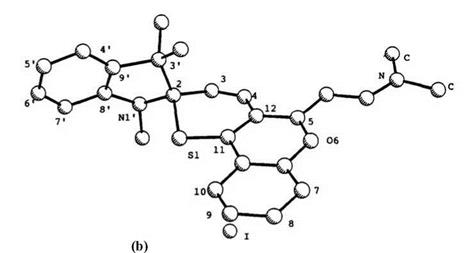
44a (44: R1 = H, R2 = 6-NO2) and 45b are listed in Table 8 and their PLUTO figures are shown in Figure 1.10.91
The C—S bond length (1.87 or 1.89A) between the sulfur and the spiro carbon in both spirothiopyrans is longer than found for the noncon — jugated C—S bond (1.77A), and longer than normal C—S bonds in benzothiopyrans. The nitro-substituent in the indoline component affects the structure of spirothiopyrans. In the series of spirobenzothiopyrans 44, the double bond length (C3-C4) in the thiopyran ring for 5′-nitro derivative 44b (44: R1 = NO2, R2 = 6-NO2) becomes shorter (0.08 A) and the N1′-C8′ bond length becomes shorter (0.03 A). On the other hand, in a
|
|
|
Figure 1.10. PLUTO views and numbering of spiro compounds: (a) 44a; (b) 45b. |
|
Table 9. Deviations from the Optimum Plane 1 in 44a and 44b Deviations (A)a
“Estimated standard deviations: 0.005-0.009 A. |
series of spirothiopyrans 45, this substituent effect leads to shorter Nl’-C8′ bonds.
The heterocyclic rings in the indoline component in 44 and 45 are not planar, but the deviations from the optimum planes of this heterocyclic ring are affected by nitro substituents. This deviation in 5′-nitro derivative 44b is smaller, compared with 44a as shown in Table 9. The dihedral angles between the indoline ring (plane 1) and benzene ring (plane 2) fused to the indoline ring are 7.31° and 2.05° for 44a and 44b, respectively, indicating an increase in planarity of the indoline component due to the nitro substituent in 44. In the spirothiopyran series 45, similar increase in this planarity is observed since the means deviation from the plane of the indoline ring (plane 1) decreases from 0.143 A to 0.122 A and the dihedral angle between the indoline ring and the benzene ring fused on the plane 1 ring also decreases from 9.55 to 6.09°.
 4 июля, 2015
4 июля, 2015  Malyar
Malyar 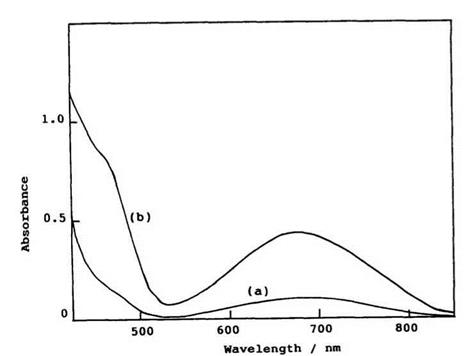
 Опубликовано в рубрике
Опубликовано в рубрике 