As one of the main synthetic routes to tetrazolium salts, the oxidation of formazans has been discussed in Section 1.3.1.5. Strong oxidizing agents such as concentrated nitric acid and singlet oxygen (produced chemically or with a sensitizer of low triplet energy such as methylene blue) can lead to degradation to benzoic acid, phenol, and benzene and have no synthetic utility. When a sensitizer with high triplet energy, such as Eosin Y, is used, the expected tetrazolium salt can be isolated.358
Verdrazyls, e. g., 210, are a class of cyclic free radicals, with characteristic ESR spectra and intense colors. They represent a special case of “oxidation” of formazans and can be obtained from the methylation of and simultaneous or subsequent dehydrogenation of formazans.359-361 Ver-
|
drazyls can undergo further oxidation to formyl formazans, e. g., 211 (Scheme 34). Polymers containing stable verdrazyl radicals have been prepared and suggested as potential semiconductors 362,363
 5 октября, 2015
5 октября, 2015  Malyar
Malyar 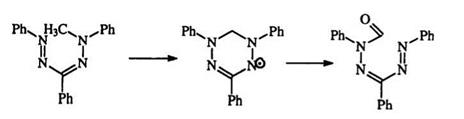
 Опубликовано в рубрике
Опубликовано в рубрике 