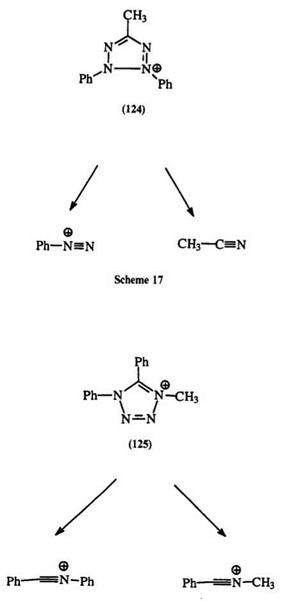
NMR spectroscopy is commonly used for the identification of isomeric tetrazolium salts. There are significant differences in the chemical shifts of C-methyl and ^-methyl protons as shown in Table 4 for compounds 128
![]()
|
|
||
|
|
||||||||||||||||||||||||||||||||||||
 |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
|
|
Compound |
X |
1-CH3 |
3-CH3 |
4-CH3 |
|
136 |
H |
4.40 |
4.40 |
|
|
136 |
no2 |
4.45 |
— |
4.45 |
|
137 |
H |
4.60 |
4.80 |
— |
|
137 |
no2 |
4.65 |
4.90 |
— |
 |
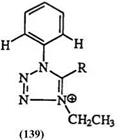 |
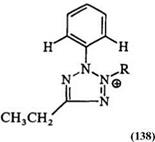
Table 7. ‘H-NMR Chemical Shifts (5) of N-Phenyl Tetrazoliums 138 and 139
|
Compound |
R |
H(R) |
О ffi |
О ffi |
H(Ph-2’/6′) |
|
138 |
H |
1 1 .30 |
1.80 |
4.96 |
8.02 |
|
138 |
CH3 |
2.93 |
1.73 |
4.73 |
7.75 |
|
138 |
CH2C1 |
5.31 |
1.76 |
4.88 |
7.78 |
|
139 |
H |
2.93 |
1.82 |
5.07 |
8.26 |
|
139 |
CH2C1 |
5.45 |
1.80 |
5.03 |
8.24 |
 |
|
Compound |
H-5 |
Ph-2′-H |
Ph-3 ‘/14’-H |
|
140 |
10.74 |
7.81 |
7.56 |
|
141 |
10.26 |
8.26 |
7.81 |
|
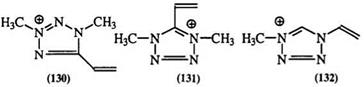
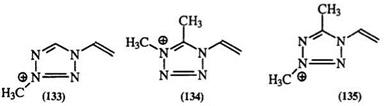
Table 9. 13C-NMR Chemical Shifts (5) of Methyl-Substituted Tetrazoliums 130-135
|
|
Table 10. |
15N-NMR Chemical Shifts (5) of Methyl-Substituted Tetrazoliums 130-132 |
|||
|
Compound |
N1 |
N2 |
N3 |
N4 |
|
130 |
-71.5 |
— 101.2 |
— 17.5 |
— 148.7 |
|
131 |
-148.1 |
-17.9 |
— 101.2 |
-76.1 |
|
132 |
-127.5 |
-22.2 |
— 14.4 |
— 142.5 |
and 129,187 Table 5 for 130—135,219 and in Table 6 for 136 and 137.180-215 The 5-ring proton chemical shifts in 5-unsubstituted tetrazolium as well as the ortho proton in phenyl substituents at the 5-position are sensitive to N-substituents and substitution patterns as shown in Table 7 for 138 and 139 and in Table 8 for 140 and 141.181-218
Carbon-13 NMR has been used in the study of thiolate and disub — stituted tetrazolium derivatives.217-219 The chemical shifts of both the substituent and ring carbons show strong sensitivity to substitutents as shown in Table 9 for 130—135.219 Tetrazolium salts have also been studied by nitrogen-15 NMR (Table 10).220’221
 23 сентября, 2015
23 сентября, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 