The X-ray crystal structures of some spiroindolinobenzopyrans, e. g., 8-NO2-BIPS and 6-NO2-8-Br-BIPS, have been determined by Russian researchers.6,7 Recently, X-ray crystal structures of the colorless form (7a) and the colored form (7b) of 6,8-dinitro-BIPS have been reported by Nakatsu8 (Figure 1.3).
In the colorless form (7a), the C2—O1 bond length (1.50A) is significantly longer than 1.43A found for the normal C—O bond length in benzopyran. The dihedral angle between an O1—C2—C33 plane and an N1′-C2′-C3′ plane in two heterocyclic rings is ca. 90°, and heterocyclic rings are almost perpendicular to each other; nevertheless, they are not planar. The distance (2.68 A) between the oxygen atom in the benzopyran and the carbon atom of methyl group at the 3′-position in the indoline ring is significantly shorter than the van der Waals radius for such atoms. This steric effect may aid in the ring opening. This C2—O1 bond extension and steric effect are similarly observed in spirothiazolinobenzopyran.9 From these observations, it is suggested that the C2—O1 bond is very weak and may easily be cleaved.
In the colored form (7b), the N1’—C2 bond length in the indolinium component is shorter than for the colorless form, indicating the double bond character. The C9—C10 bond length becomes longer than other C—C bonds in the phenolate ring. Alternation of the C—C bond length from C2 to C10 is reduced in the colored form. The strong interaction between phenolate oxygen with C3 or oxygen atom in the nitro group in the colored form is suggested, because of their shorter distances (Figure 1.3). In the solid state, the C9—O1 bond can be regarded as a carbonyl group. Thus, the molecular geometry in the colored form for 7 consists of an indolinium cation and negative phenolate ring. Therefore, suggestion of the polar
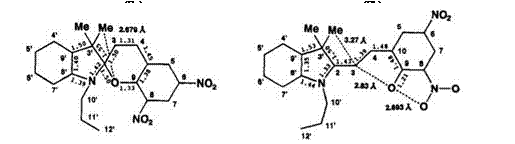
zwitterion form is reasonable. However, the C—C bond in the conjugated chain is not always homogeneous in the merocyanine form for other spirobenzopyran series. For example, the C3—C4 bond is remarkably short (1.30-1.35 A) in the photomerocyanine form of spirobenzothiazolinoben — zopyran10 and spiroindolinoxazolidine.8
 21 июня, 2015
21 июня, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 