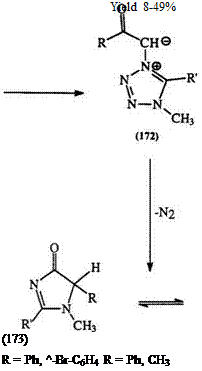
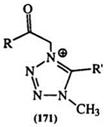
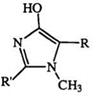
The methyl hydrogens in 1,3,5- and 1,4,5-trimethyl tetrazoliums, as well as the proton on C-5 in 2,3-disubstituted tetrazoliums are acidic, and can be abstracted with butyl lithium.289,290 Tetrazolium methylides, e. g., the dicyano derivative (143), and the phenacyl compound (171, R = Ph, R’ = Me) are known.291 The latter undergoes an unexpected thermal cyclization reaction to yield imidazolones (173) (Scheme 27).292
 |
|
 |
 |
Tetrazolium ylides are quite reactive and are easily alkylated.168 The mesoionic tetrazolium thiolate 117 readily adds bromine to yield 174 which can then react with a number of active methylene compounds to give mesoionic compounds, e. g., 175.293,294 They also undergo 1,3-dipolar cycloaddition with olefins and acetylenes to yield bicyclic tetrazolo-thiazolines
|
|
||
|
|||
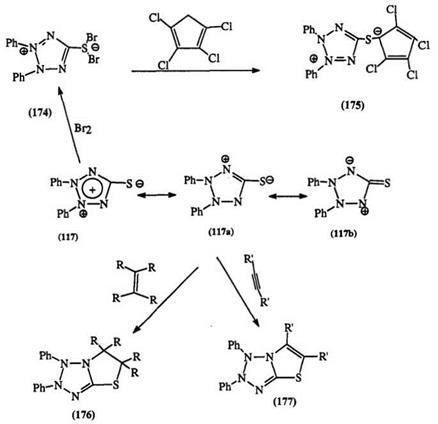
(176) and tetrazolo-thiazoles (177), respectively (Scheme 28).216,295 They react across the S—C—N dipole of hybrid 117a rather than the N—C—N dipole of hybrid 117b216 commonly seen in other mesoionic compounds.297,298 By contrast, the alkylated mesoionic tetrazolium salts react readily with reactive methylene compounds to yield conjugated mesoionic conounds, e. g., 144.296
The chemistry of tetrazolium thiolates and other mesoionic tetra — zoliums has been extensively reviewed.297,298 Tho ugh there are no reports of the chemical reduction of thiolates to formazan-like structures, the polarographic reduction of the complex betaines (146) to formazans has been reported.655
 29 сентября, 2015
29 сентября, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 