The spiropyran gives the colored merocyanine form by photodissociation of the spiro C—O bond. Generally, the ring-open reaction of spiroin — dolinobenzopyrans proceeds via the following mechanism: The weakening of the C—O bond due to photochemical activation of the л-electron system of the spiropyran (SP*)1 (Scheme 5), and the dissociation of C—O bond depends on the interaction of the electrons of the nitrogen-nonbonding orbital and the unoccupied antibonding orbital (a*) of the spiro C—O bond. The electron-donating substituent group in the spiroindolinoben — zopyrans has a tendency to increase the polarization of the C—O bond.
In the case of spiroindolinobenzopyrans without a nitro group, the photocoloring reaction generally proceeds via the excited singlet state of the
|
|
molecule, and then formation of the cis-cisoid isomer X (A in Figure 1.1). Finally, a cis-trans isomerization gives a transoid colored form (CF) (Scheme 5). For spiropyrans having an extended aromatic n system such as spiroin- dolinonaphthopyrans the photocoloring reaction also proceeds via the singlet state. The cis-cisoid isomer X has been proposed to be an intermediate in the isomerization of spiropyrans containing a heterocyclic component such as indoline, oxazine, and thiazine.26’27 Such short-lived intermediate (lifetime 10-s-10-3s; Xmax 430-450 nm) in photocoloring reaction has been detected by the pulse spectroscopic technique.28-30
The photocoloring reaction for spiroindolinobenzopyrans with a nitro group proceeds mainly via the formation of the excited triplet state of the molecule. The reaction proceeds partly from the triplet state [(SP*)3] of the spiropyran to the triplet state (X)3 of the cis-cisoid isomer which subsequently transforms into the CF and partly from (SP*)3 to the CF. This process from (X)3 to the colored form is accelerated by the presence of atmospheric oxygen (Scheme 6).2,28 For the photocoloring reaction, the participation of singlet or triplet state depends not only on the substituent but also on the nature of the heterocyclic component.
The quantum yields for photocoloration of spirobenzopyrans are collected in Ref. 1. Generally, the coloration quantum yields of spiroindolinobenzopyrans by UV irradiation (366 nm) at room temperature (15- 25°C) are 0.1-0.7. Photobleaching quantum yields by visible light are very small (<0.1) and less accurate, since both thermal and photobleaching occur simultaneously.
The nitro substituent has significant influence on photofading of spiroindolinobenzopyrans and the BIPS systems. Without a nitro group, they show better stability to the light. The electron-withdrawing substituent in spirobenzopyrans decreases the polarization of the C—O bond, leading to less likely homolitic cleavage of the C—O bond. This is largely responsible for the degradation of the spirobenzopyrans. 1,31 The solvent also has a direct influence on the polarization of the C—O bond. The spirobenzo — pyrans show better stability to the UV light when the solvents have a high
|
|
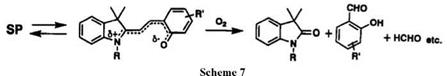
degree of solvation (polar solvent).2 Photofading of spiroindolinoben — zopyrans in aerated solution produced partially oxidized products, such as salicylaldehyde derivative, oxindole, formaldehyde, and oxidized derivatives of solvents (Scheme 7).32-34
 24 июня, 2015
24 июня, 2015  Malyar
Malyar 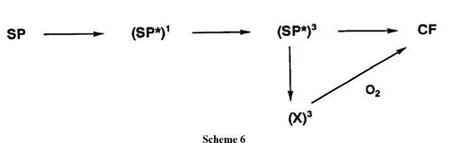
 Опубликовано в рубрике
Опубликовано в рубрике 