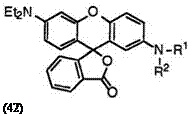
Fluoran compounds having two tertiary amino groups at 2′- and 6′-positions generally develop green color. For example, 2′,6′-bis(diethyl- amino)fluoran (42; R1, R2 = C2H5),5 2′-(N-benzyl-N-ethylamino)-6′-di — ethylaminofluoran (42; R1 = C2H5, R2 = C6H5CH2),5 2′-dibenzylamino-6′- diethylaminofluoran (42; R1, R2 = C6H5CH2),5 6′-diethylamino-2′-diphen- ethylaminofluoran (42; R1, R2 = C 6H5C2H4),34 6′-diethylamino-2′-(N — methylanilino)fluoran (42; R1 = CH3, R2 = C6H5),36 2′-dibenzylamino — 6′-(N-ethyl-4-methylanilino)fluoran (43; R1, R2 = C6H5CH2),22 6′-(N-
ethyl-4-methylanilino)-2′-(N-methylanilino)fluoran (43; R1 = CH3, R2 = C6H5),22 2′-diallylamino-6′-(N-ethyl-4-methylanilino)fluoran (43; R1, R2 = CH2=CHCH2),37 2′-di-«-propylamino-6′-(N-ethyl-4-methylanilino)fluoran
(43; R1, R2 = n-C3H7),37 2′-dicinnamylamino-6′-(N-ethyl-4-methylanilino)- fluoran (43; R1, R2 = C 6H5CH=CHCH2),37 etc. develop green color.
|
(43) |
 |
 |
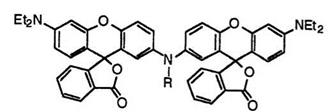
In addition, 2′,2’"-N-ethyliminobis(6′-diethylaminofluoran) (44; R = C2H5),38 2′,2’"-N-benzyliminobis(6′-diethylaminofluoran) (44; R = C6H5- CH2),38 2′,2’"-N-cinnamyliminobis(6′-diethylaminofluoran) (44; R = C6H5- CH=CHCH2),38 2′,2’"-N-propargyliminobis(6′-diethylaminofluoran) (44;
R = CH=CCH2),38 etc. develop green color.
![]()
|
|
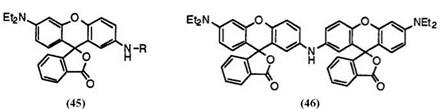
When secondary amino groups are employed in the place of tertiary amino groups at 2′-position, the fluoran compounds develop dark green or greenish black color. These include 2′-n-butylamino-6′-diethylaminofluoran (45; R = n-C4H9),5 6′-diethylamino-2′-n-octylaminofluoran (45; R = n — C8H17),39 2′-allylamino-6′-diethylaminofluoran (45; R = CH2=CHCH2),35 2′-benzylamino-6′-diethylaminofluoran (45; R = C6H5CH2),5 6-diethyl — amino-2′-phenethylaminofluoran (45; R = C6H5C2H4),20 2′-cinnamyl — amino-6-diethylaminofluoran (45 R = C6H5CH=CHCH2),35 2′-anilino-6′- diethylaminofluoran (45; R = C6H5),39 and 2′,2’"-iminobis(6′-diethylamino — fluoran) (46).38
|
|
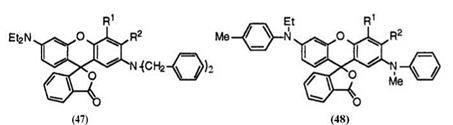
Introduction of an additional substituent such as alkyl, alkoxy, halogen, etc. at 3′-position has a large effect on color. That is, green color changes to red color when there is a tertiary amino group at 2′-position. Thus, 2′-dibenzylamino-6′-diethylamino-3′-methylfluoran (47; R1 = H, R2 = CH3 ),40 2′-dibenzylamino-6′-diethylamino-3′-ethylfluoran (47; R1 = H, R2 = C2H 5),34 3 ‘-chloro-2′-dibenzylamino-6′-diethylaminofluoran (47; R1 = H, R2 = Cl),34 6′-(Wethyl-4-methylanilino)-3′-methyl-2′-(Wmethylanilino)flu — oran (48; R1 = H, R2 = CH 3),22 etc. develop red color. If there is a secondary amino group at 2′-position, black color can be obtained: this will be discussed in Section 6.2.2.6. On the other hand, introduction of an additional substituent at 4′-position exerts little influence on color. Thus, 2′-dibenzylamino-6′-diethylamino-4′-methylfluoran (47; R1 = CH3, R2 = H),38 4′-chloro-2′-dibenzylamino-6’-diethylaminofluoran (47; R1 = Cl,
R2 = H),38 2′-dibenzylamino-6′-diethylamino-4′-methoxyfluoran (47; R1 = CH3O, R2 = H),41 4′-chloro-6′-(N-ethyl-4-methylanilino)-2′-(N-methylani — lino)fluoran (48; R1 = Cl, R2 = H),22 6′-(N-ethyl-4-methylanilino)-4′-methyl- 2′-(N-methylanilino)fluoran (48; R1 = CH3, R2 = H),22 etc. still develop green color.
|
|
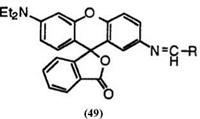
Replacement of amino groups at 2′-position with azomethine groups gives brown color. These include 6′-diethylamino-2′-ethylidenamino — fluoran (49; R = CH3),42 2′-(2-butenylidenamino)-6′-diethylaminofluoran (49; R = CH3CH=CH),42 2′-benzylidenamino-6′-diethylaminofluoran (49; R = C6H5),42 and 2′-cinnamylidenamino-6′-diethylaminofluoran (49; R = C6H3CH=CH).42
|
|
 27 августа, 2015
27 августа, 2015  Malyar
Malyar 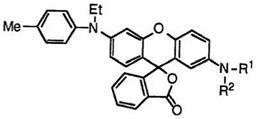
 Опубликовано в рубрике
Опубликовано в рубрике 