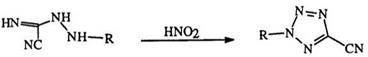
Nitrous acid or alkyl nitrites react with a number of nitrogen compounds to yield tetrazoles. For example, hydrazidines (87), which can be prepared in situ from the corresponding iminoesters, react with nitrous acid or its derivatives to give 1-substituted tetrazoles (88).150152 This reaction (Eq. 17), is one of the most extensively used methods for the synthesis of
|
|
|
|
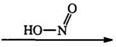
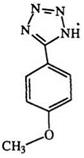
many substituted tetrazoles. It is worth noting here that the first reported synthesis of an amino tetrazole involved the diazotization of amino- guanidine.153 Diazotization of 1-cyanoformimidic acid hydrazide (89) yields 5-cyanotetrazole (90).154 The diazotization of the phenyl derivative [(89) R = Ph], obtained from the reaction of cyanogen and phenylhyd — razine, was in fact the basis of the first recognized synthesis of a tetrazole ring (Eq. 1 8).155,156 1-Alkyl-2-aminoguanidine (91) (R2 = H) is diazotized to two isomeric tetrazoles 92 and 93 in which the 5-amino-1-alkyl isomer (92) predominates.117 However, disubstituted aminoguanidines (R2 = alkyl
|
R = H, Alkyl or Aryl
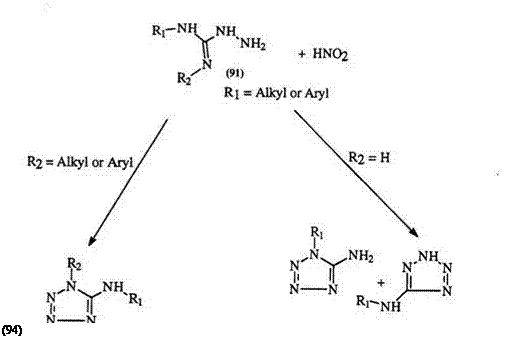
Scheme 12
or aryl) yield the corresponding 5-aminotetrazoles (94) only (Scheme
12).157,158
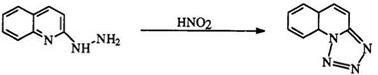
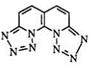
Fused ring tetrazoles such as 96 are obtained from the reaction of nitrous acid with heterocyclic hydrazines (95) (Eq. 19).159,160 This method is also suitable for the preparation of fused ring ditetrazoles such as
97.161 — 165
|
|
|
(97) |
 16 сентября, 2015
16 сентября, 2015  Malyar
Malyar 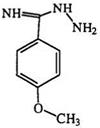
 Опубликовано в рубрике
Опубликовано в рубрике 