1,5- Disubstituted tetrazoles are conveniently prepared from acyl hydrazines (98) and diazonium salts.166 The reaction proceeds through the intermediate tetrazenes (99) followed by cyclization to the tetrazole (100) (Scheme 13). The intermediate can be isolated under mildly basic conditions. Symmetrically 1,2-diacylated hydrazines yield 1-substituted tetrazoles through the elimination of one of the acyl groups.166 — 168 Diformyl — hydrazine is a very convenient starting material for 1-substituted tetrazoles.166′ Unsymmetrically 1,2-diacylated hydrazine usually results in mixtures.169
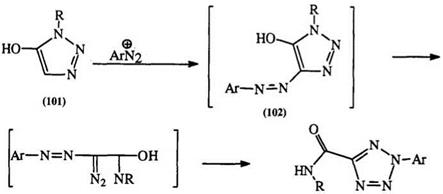
Diazonium salts couple to hydroxy-substituted vicinal triazoles (101) with subsequent rearrangement of the hydroxy arylazo compounds (102) to the carbamoyl tetrazole (104).170 An open-chain intermediate (103) has been proposed.169 This rearrangement is similar to that of the benzoyl
|
|
|
|
||
|
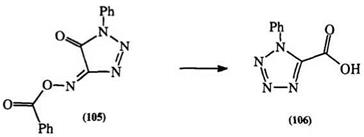
derivative of isonitroso triazolinone (105) to the tetrazole (106) (Scheme 14).i7i — i74 3_Amino isoxazoles also rearrange to tetrazoles through a triazine intermediate.173,174
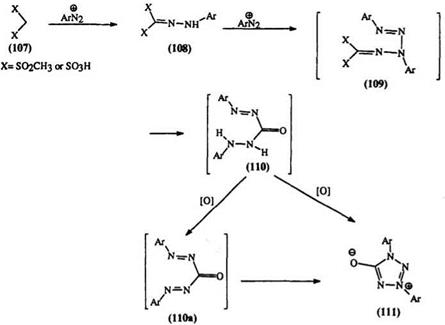
Diazonium salts react with bis(methylsulfonyl) methane (107) (X = SO2CH3) to yield a 1,3-diaryl tetrazolinone (111). The reaction proceeds through an azo (108) and a tetrazene (109) intermediate, followed by hydrolysis under the alkaline conditions of the reaction to the carbonyl compound (110). An unexplained oxidation leads to the 1,3- diaryl tetrazolinone (111) either directly or through the intermediate 110a (Scheme 15).18,35 A similar reaction occurs between a diazonium salt and the potassium salt of phenyl hydrazonomethane disulfonic acid (Scheme15).175
|
Scheme 15 |
 16 сентября, 2015
16 сентября, 2015  Malyar
Malyar 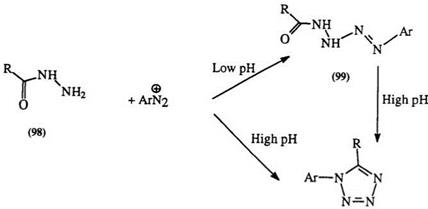
 Опубликовано в рубрике
Опубликовано в рубрике 