Absorption spectra of formazans have been studied in detail. Almost all formazans exhibit UV/visible spectra between 300 and 600 nm.1’2’12’13’40 62’325’326 The absorption maxima are very sensitive to substituent effects. For example, the 1,5-diphenyl formazan 185 when X is hydrogen, methyl, phenyl, cyano, and mercapto shows a band at 420, 410, 470, 504, and 590nm in ethanol, respectively. The 3-chloro derivative 186 when X is hydrogen, iodine, bromine, chlorine, and fluorine has a band at 433,433,430,421, and 417 nm, respectively. Table 13 shows the influence of substituents on the absorption maxima in the trisubstituted formazans 3. Table 14 shows the influence of substituents on the absorption maxima of
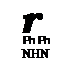 |
(185)
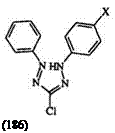 |
a number of 1-(2-benzothiazolyl)-substituted 3,5-diaryl formazans 187.283 A plot of the absorption frequencies of the formazan dyes versus the Hammett sigma values of the substituent on the 3-aryl ring is linear; a similar plot for the 5-aryl ring results in a parabolic-shaped curve with a peak at a sigma value of zero hydrogen. Similar results are observed with other heterocyclic
f 231 319 — 324 331
lormazans. ’ 324,331
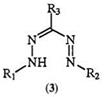 |
Table 13. Absorption Maxima of Substituted Formazan (3)
|
R1 |
R3 |
R2 |
^max(nm) |
|
Ph |
H |
Ph |
260,285,420 |
|
Ph |
CH3 |
Ph |
260,410 |
|
Ph |
n-C11 H22- |
4-NO2-C6H4 |
427 |
|
Ph |
Ph |
Ph |
405 |
|
Ph |
Ph |
a-Naphthyl |
275,520 |
|
Ph |
Ph |
P-Naphthyl |
270,305,505 |
|
Ph |
Ph |
4-Biphenyl |
410 |
|
Ph |
4-Biphenyl |
Ph |
320, 500 |
|
4-Biphenyl |
Ph |
4-Biphenyl |
435 |
|
Ph |
Ph |
4-C1-C6H4 |
290,410 |
|
Ph |
Ph |
4-I-C6H4 |
500 |
|
4-I-C6H4 |
Ph |
4-I-C6H4 |
510 |
|
a-Naphthyl |
Ph |
4-NO2-C6H4 |
290, 365,438 |
|
4-NO2 — C6 H4 |
Ph |
4-I-C6H4 |
500 |
Table 14. Absorption Maxima of Substituted Formazan (187)
|
|
|
X |
Y |
XCHC13 |
XDMF |
|
H |
H |
416 |
539 |
|
4-NO2 |
H |
542 |
623 |
|
4-C1 |
H |
563 |
632 |
|
4-CH3 |
H |
578 |
640 |
|
4-OCH3 |
H |
584 |
646 |
|
4-Br |
H |
562 |
635 |
|
3-Br |
H |
555 |
630 |
|
3-OCH3 |
H |
564 |
640 |
|
3-NO2 |
H |
546 |
623 |
|
2-NO2 |
H |
492 |
626 |
|
2-C1 |
H |
456 |
630 |
|
2-CH3 |
H |
451 |
637 |
|
2-OCH3 |
H |
454 |
642 |
|
H |
no2 |
570 |
563 |
|
H |
Cl |
484 |
550 |
|
H |
CH3 |
416 |
537 |
|
H |
OCH3 |
416 |
537 |
|
H |
CF3 |
535 |
560 |
|
H |
CN |
554 |
584 |
|
H |
N(CH3)2 |
514 |
514 555 (DMF/NaOH) |
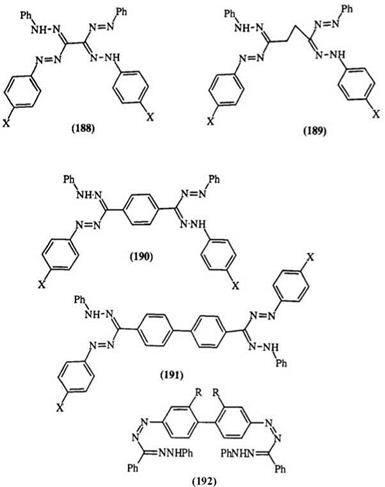
Bis-1,5-diaryl formazans generally absorb at longer wavelengths than mono formazans. The absorption maxima depend on the linking group. Formazans 188—191 (X = H) absorb at 485,470, 520, and 572 nm, respectively. The absorption maxima are also influenced by substituents. The formazan 190 (X = CO2Et) absorbs at 510 nm whereas 191 (X = Me, OMe) absorbs at 585 and 606 nm, respectively.12,13 The absorption maximum of 192 shifts from 572 (R = H) to 585 and 602nm (R = Me, OMe), respec-
|
|
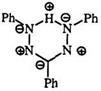
tively.327 The substituent effects are explained on the basis of a pseudoaromatic structure 193 with alternating polarity. 197,328 Huckel molecular orbital calculations of the electron densities on the five atoms in symmetrically substituted formazans support this view.329 The agreement is not satisfactory for unsymmetrically substituted formazans.330 The electronic spectra of formazans have been the subject of molecular orbital calculations using the LCAO method331 and the SCF method.332 The latter method has been extended to study the thermal and electronic conductance properties of 1,5-diphenyl-3-cyanoformazan.333 Bond distances calculated using the PPP method correlate well with X-ray data.315
|
(193) |
The spectral absorptions shift to longer wavelengths as the solvent polarity increases. However, care must be taken to distinguish them from the spectral shifts due to deprotonation.
 2 октября, 2015
2 октября, 2015  Malyar
Malyar 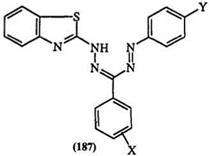
 Опубликовано в рубрике
Опубликовано в рубрике 