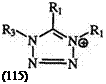
Some of the methods used for the preparation of either formazans or tetrazoles can lead directly to tetrazolium salts when appropriate substituents are present.
7.3.3.1. From Nitrilium Salts
Nitrilium salts (66) react with alkyl or aryl azides to give good yields of 1,4,5-trisubstituted tetrazolium salts (115) (Eq. 20).189,255
7.3.3.2. Oxidations
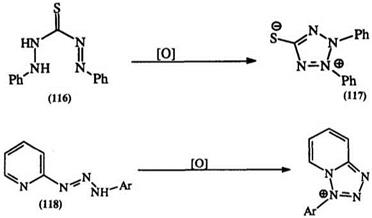
Ferricyanide oxidation of 1,5-disubstituted thiocarbazones (116) give the mesoionic tetrazolium salts (117) under mild conditions (Eq. 21).190 This is in contrast to the strongly alkaline oxidation of carbazides leading to mercapto formazans as shown in Eq. 8 (Section 7.3.1.4). The heterocyclic triazine (118), obtained by the action of a diazonium salt on 2-
Ф
 Ri—C=N—R2 + R3—N3
Ri—C=N—R2 + R3—N3
![]() (20) (66)
(20) (66)
Rl, R2 =Alkyl or Aryl
|
R1 |
R2 |
R3 |
Yield (%) |
|
CH3 |
CH3 |
CH3 |
80 |
|
CH3 |
CH3 |
CH2Ph |
71 |
|
CH3 |
C2H5 |
C2H5 |
58 |
|
Cyclohexyl |
CH3 |
CH2Ph |
60 |
|
1-D4H9 |
CH3 |
CH2Ph |
60 |
|
Ph |
CH3 |
CH3 |
75 |
|
Ph |
CH3 |
CH2 Ph |
70 |
|
Ph |
C2H5 |
C2H5 |
55 |
|
Ph |
Ph |
CH3 |
78 |
|
Ph |
Ph |
CH2Ph |
65 |
|
Ph |
Ph |
Ph |
52 |
 (21)
(21)
aminopyridine, is oxidized by the action of tribromophenol-bromine in ethyl acetate at room temperature to the fused tetrazolium salt (119) in excellent yield (78—92%) (Eq. 22).191
Synthesis of 2-Benzyl-1,5-dimethyltetrazoliumfluorosulfonate (115b). 189 An equimolar mixture of acetonitrile and methyl fluoro-sulfonate was kept
|
|
at room temperature for 5 h. The resulting solid was washed several times with anhydrous ether and filtered. The product (75% yield), mp 123— 124 °C, was used without further purification.
To the above product, dissolved in acetonitrile, was added an equimolar amount of benzyl azide and the solution stirred under reflux for 12 h. On cooling, the precipitated product was collected, washed with ether, and dried to yield 71% of the pure product.
Synthesis of 3-Phenyltetrazolopyridinium bromide (119). 660 To a solution of 2.88 g of 1-(2-pyridy1)-3-phenyltriazine was added 4.92 g of 2,4,4,6- tetrabromocyclohexa-diene-1-one. The product precipitated within minutes and was isolated by filtration in 87% yield mp 278—280 °C.
 18 сентября, 2015
18 сентября, 2015  Malyar
Malyar 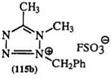
 Опубликовано в рубрике
Опубликовано в рубрике 