In order that diarylmethane phthalides function as color formers, it is necessary to replace the hydrogen atom in the 3-position of the phthalide ring by an electronegative group. Scheme 5 typifies the synthetic route to such phthalides. Once again the key intermediate is the carboxyben — zophenone which, on treatment with acetic anhydride, yields the 3- acetoxyphthalide.40 Treatment with alkylamines,41 anilines,42 or diphenyl — amines43 leads to the formation of the corresponding 3-amino-substituted phthalides. Finally, ethers and thioethers have also been reported,44 being obtained by reaction of the benzophenone first with thionyl chloride followed by an alcohol, phenol, or thiophenol.
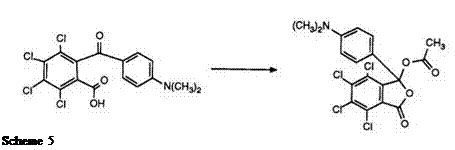
All of these phthalides in which the phthalide ring is not substituted by electron-donating groups are yellow to orange color formers, and useful as
|
Scheme 6 |
shading components for the production of black images. However, introduction of a dialkylamino group into the 6-position of the phthalide ring results in bluish-green shades.
 30 июля, 2015
30 июля, 2015  Malyar
Malyar 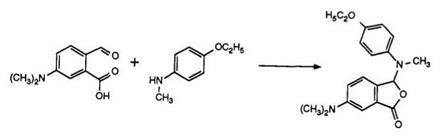
 Опубликовано в рубрике
Опубликовано в рубрике 