3.5.1.1. Acid—Base Properties
Formazans behave as weak acids as well as weak bases. Salts of formazans have been isolated.26’334’335 The acid dissociation constants of some substituted formazans have been determined from their solution spectra.336
Though formazans can be protonated, there are no reports of isolation of formazan cations. The study of the basicity of formazans is complicated by the fact that exposure to acid can lead to irreversible chemical changes. Recently, the protonation of the triaryl formazan 194 with perchloric acid in aprotic solvents has been studied spectroscopically. In this a hypso— chromic shift is observed and is more pronounced when X is an electron— donating substituent. Thus, the shift is 33, 14, and 73nm when X is hydrogen, j9—methoxy, and те—nitro, respectively.337
|
|
R = PhN=N
 |
 |
= Alkyl or Aryl
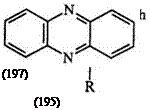
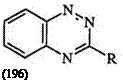
Scheme 29
1.52.2. Reaction with Acids
In warm mineral acid, formazans (195) rearrange to a benzotriazine (196) eliminating an amine (Scheme 29).8’26’21’334’338’339 H owever, when R is methyl, phenylazo, or oxalyl, the main product is the phenazine 197 along with a trace amount of the benzotriazine.340 The mechanism of this reaction is not well understood.341 -345
 2 октября, 2015
2 октября, 2015  Malyar
Malyar 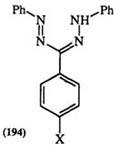
 Опубликовано в рубрике
Опубликовано в рубрике 