4.7.1. Spirofluorene Phthalides
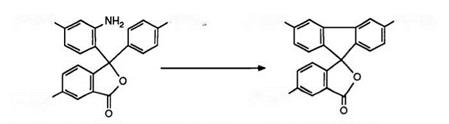
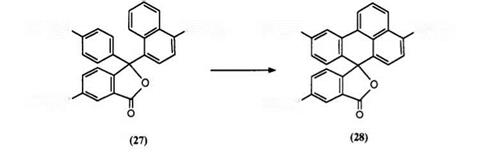
Fusion of the two arylamino groups of a triarylmethane phthalide color former results in the formation of spirofluorene phthalides. Due to the increased planarity of this system, a bathochromic shift results leading also to color formers showing infrared absorption when developed. This was first exemplified in 1983102 by preparation of phthalide 26 as shown in Scheme 10.
The phthalide 25, obtainable by condensation of 4,4′-bisdimethyl — aminobenzophenone-2-carboxylic acid with 3-dimethylaminoacetanilide
and subsequent hydrolysis, was diazotized in sulfuric acid and the resultant diazonium salt treated with copper powder to yield 26. However, better yields are reportedly obtained by carrying out ring closure of the diazonium salt in phosphoric acid.103 A further synthetic route has also been described in which phthalides undergo intramolecular cyclization in the presence of aluminum chloride and urea.104,105 Thus, Crystal Violet lactone (2) has been directly converted into phthalide 26.106
Despite the fact that the first patent application102 also claimed preparation and use of azaphthalides, such compounds never, in fact, appear
![]()
|
|
||
|
|
||
|
|||
|
|||
|
|
Scheme 10
to have been synthesized, and the only variations on the basic structure reported to date concern differing substituents on the amino groups. Thus, for example, alkoxyalkylamino and phenoxyalkylamino substituted analogues of 26 are claimed to possess superior stability toward heat after development,107 but further-reaching structural variations have not been reported.
 5 августа, 2015
5 августа, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 