The most remarkable feature of fluoran compounds is producing singly black color, which can hardly be attained by any other class of leuco dyes.
One of the typical black developing fluoran compounds is 2′-anilino-6′- diethylamino-3′-methylfluoran (50)6 in which the methyl group at 3′-posi — tion plays a very important role. The parent structure of 50 is 2′-anilino-6′-
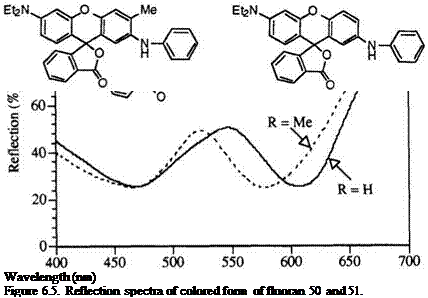 |
diethylaminofluoran (51), which colored form has two distinctive absorption maxima at 470 nm (yellow in color) and 610 nm (blue in color) in the visible region resulting in green color by the additivity of the two colors yellow and blue (Figure 6.5).
In fluoran 50, the methyl group at 3′-position causes steric hindrance to the adjacent anilino group resulting in torsion of the anilino group from the xanthene plane. Consequently, electron transfer from the anilino group to the xanthene moiety is hindered more or less, resulting in hypsochromic shift of the absorption at 610 nm to 570 nm (violet in color). On the other
hand, the absorption at 470 nm is scarcely affected by the introduction of the methyl group. The two colors yellow and violet are complementary, and fortunately the two absorption maxima are nearly the same in absorbancy. Thus, 50 develops black color by the additivity of the complementary colors of yellow and violet (Figure 6.5).
Besides the methyl group, a chlorine atom at 3′-position gives the same effect. Thus, 2′-anilino-3′-chloro-6′-diethylaminofluoran (52)43 also develops black color for the same reason.
|
|
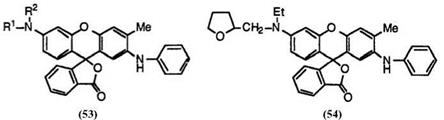
Today, many black developing fluoran compounds having a wide variety of substituents on the amino group at 6′-position are available. These include 2′-anilino-3′-methyl-6′-(N-methyl-N-n-propylamino)fluoran (53;
R1 = CH3, R2 = n-C 3H7),44 2′-anilino-6′-(N-cyclohexyl-N-methylamino)-3′- methylfluoran (53; R1 = CH3, R2 = c-C6H11),45 2′-anilino-6′-(N-ethyl-N — isobutylamino)-3′-methylfluoran (53; R1 = C2H5, R2 = i-C4H9),46 2′-
anilino-6′-(N-ethyl-N-isopentylamino)-3′-methylfluoran (53; R1 = C2H5, R2 = i-C5H11),47 2′-anilino-6′-[N-(3-ethoxypropyl)-N-ethylamino]-3′-meth — ylfluoran (53; R1 = C2H5, R2 = C2H5OC3H6),48 2′-anilino-6′-(N-ethyl-4- methylanilino)-3′-methylfluoran (53; R1 = C2H5, R2 = 4-CH3C6H4),22 2′- anilino-6′-(N-ethyl-N-tetrahydrofurfurylamino)-3′-methylfluoran (54),49 2′-
anilino-6′-di-n-propylamino-3′-methylfluoran (53; R1, R2 = n-C3H7),50
|
|
2′-anilino-6′-di-n-butylamino-3′-methylfluoran (53; R1, R2 = n-C4H9),51-52 2′-anilino-6′-di-n-pentylamino-3′-methylfluoran (53;R1, R2 = n-C5Hu),47 2′- anilino-3′-methyl-6′-pyrrolidinofluoran [53; R1, R2 = (CH2)4],53 and 2′-ani- lino-3′-methyl-6′-piperidinofluoran [53; R1, R2 = (CH2)5)].53 Each has its own characteristics regarding solubility to organic solvents, affinity with acidic compounds, etc., though all are substantially similar in color tone.
 |
Introduction of a methyl group on the anilino group at 2′-position has more than a little influence on color tone. For example, 6′-diethylamino-2′- (2,4-dimethylanilino)-3′-methylfluoran (55)54 and 6′-diethylamino-2′-(2,6- dimethylanilino)-3′-methylfluoran (56)55 develop greenish black and reddish black colors, respectively.
 |
As was described above, the hindrance of electron transfer from the anilino group to the xanthene moiety causes black color development. Therefore, besides the steric hindrance between the adjacent methyl and anilino groups, introduction of an electron-attracting group on the anilino group also causes the hindrance of electron transfer resulting in black color development. For example, 2′-(2-chloroanilino)-6′-diethyl — aminofluoran (57)56 is another typical example of black developing fluoran compounds. Additional black developing fluoran compounds having an electron-attracting group on the anilino group are 2′-(2- chloroanilino)-6′-di-n-butylaminofluoran (58; R1 = n-QH9, R2 = 2-Cl),57 6′-diethylamino-2′-(2-fluoroanilino)fluoran (58; R1 = C2H5, R2 = 2-F),58
6′-di-n-butylamino-2′-(2-fluoroanilino)fluoran (58; Ri = n-C4H9, R2 = 2- F),58 6′-diethylamino-2′-(3-trifluoromethylanilino)fluoran (58; Ri = C2H5, R2 = 3-CF3),59 and 6′-diethylamino-2′-(2-methoxycarbonylanilino)fluoran (58; Ri = C 2H5, R2 = 2-СНзОСО).20
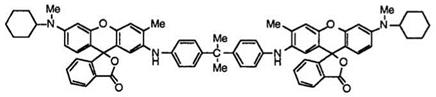
In addition, dimer-type black developing fluoran compounds
such as 2,2-bis(4-[6′-(A-cyclohexyl-A-methylamino)-3′-methylfluoran-2′-yl- amino]phenyl}propane (59)60 are also proposed. Fluoran 59 has much lower solubility in organic solvents to improve image stability to plasticizer for use in thermosensitive recording label paper.
|
(59) |
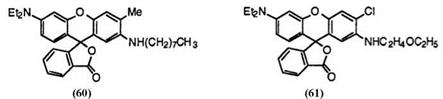
Alkylamino groups are also employed in place of anilino groups at 2′-position to give black color, though the color tone is a little greenish. These include 6′-diethylamino-3′-methyl-2′-n-octylaminofluoran (60),61 and 3′-chloro-6′-diethylamino-2′-(2-ethoxyethylamino)fluoran (61).62
|
|
 28 августа, 2015
28 августа, 2015  Malyar
Malyar 
 Опубликовано в рубрике
Опубликовано в рубрике 