Use of benzotriazole in the preparation of diphenylmethanes and triphenylmethanes has been reviewed.99 Benzotriazole is condensed with an aldehyde and then allowed to react with naphthols to form a diphenyl — methane benzotriazole derivative such as 69 (Scheme 9). The benzotriazole moiety in 69 is displaced by a Grignard reagent to give triphenylmethanes.79,100 This method allows for the preparation of triarylmethanes which contain three different aromatic rings. Compounds 70-72 are prepared by this method.
|
|
|
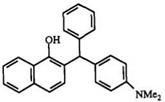
Scheme 9
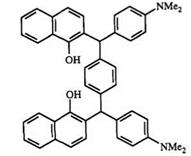
Experimental Procedure79 for 1,4-bis(4-dimethylaminophenyl) (1 — hyd — roxynaphthalen-2-yl)methylbenzene (72). To a solution of 1,4-bis(benzo — triazol-1-yl) (1-hydroxynaphthalen-2-yl)benzene (3.74 g, 6 mmol) in dry THF (240ml) at — 78°C was added a solution of 4-dimethylaminophenyl- magnesium bromide (60mmol, 1.0M in THF, 60ml). The mixture was allowed to warm to room temperature and stirred overnight. It was then poured into water (100ml), acidified with HCl (2 N), and extracted with Et2O (4 x 100ml). The ether extracts were dried (MgSO4) and concentrated to give solid. The solid was purified by column chromatography with gradient eluent (hexane/CH2Cl2 4:1, then CH2Cl2) to give product (1.42 g, 38%) as glassy solid.
 17 августа, 2015
17 августа, 2015  Malyar
Malyar 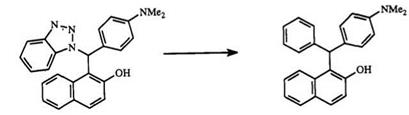
 Опубликовано в рубрике
Опубликовано в рубрике 