Leuco naphthazarins have been well studied as hair dyes.25 Human hair has been colored purplish red from dye solutions in aqueous benzyl alcohol. For example, 33 has been oxidized on hair during the drying process.
Although leuco quinones have been studied as color formers, their use in color-forming recording media has not been studied extensively due to their instability.
Recently, Yoshida and co-worker26 — 29 have developed a series of new color-forming systems using metal complexes of leuco quinones. Many bidentate ligands that produce a large bathochromic shift of absorption
maximum with an increase in molar extinction coefficient have been reported. The N, O-bidentate indoaniline-type ligand, 5-(4-dimethylamino — phenylimino)quinolin-8(5^)-one (49a)26 readily forms complexes with metal ions to produce near-infrared absorption at around 720 nm. Hence, use of metal chelate complexation together with the redox process of the dye 49 is of particular interest for developing new near-IR color-forming systems. Reduction of 49a with sodium dithionite in alkaline conditions
|
(54) a; X = CI b; X = Br |
gives the leuco dye 50a in high yield, which has weak absorption maximum at 403 nm. Leuco dye 50a can be isolated as a stable compound. This may be attributed to the formation of an intramolecular hydrogen bond between the 1-nitrogen atom and 8-hydroxy group. Addition of copper salt to an ethanol solution of 50a results immediately in the increase of absorbance at 724 nm suggesting oxidation to form the metal complex 51a. Rapid formation of an intense absorption band in the near IR region is important from the viewpoint of some color-forming systems. Such color-forming systems can be applied to labels for use with diode laser readable direct thermal printing systems. It is also notable that the rate of color development and the absorption maximum of 51 can be affected by complexing metal salts. Related leuco quinoid dyes such as 52,27 53,28 and 5429 have been reported which show similar color-developing behavior and formation of metal complexes (Scheme 18).
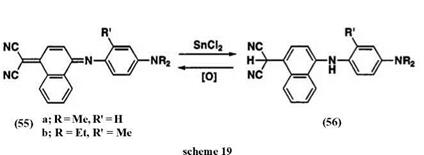
In the case of the naphthoquinone methine-type near-IR dye 55, reduction with tin(II) chloride under acidic conditions gives the leuco dye 56, which has weak absorption maxima at 350-359 nm in methanol. The leuco dye 56 can be isolated as a stable pale yellow compound. The oxidation behavior of 56 has been studied by adding benzoquinone as oxidant in methanol solution. Compound 56 immediately produced new absorption at 760 nm which is consistent with the absorption maximum of 55 (Scheme 19).30 The absorption spectra of the leuco, quinone, and metal complex forms are summarized in Table 3.
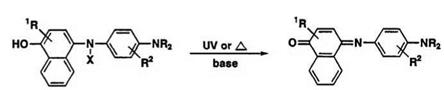
The leuco dye 57 (cf. dye 20) has been used as a photosensitive12 or a photothermographic material13 capable of producing a high-density cyan image. Dye 57 is stable enough not to be oxidized by oxygen of the air or by simple heating. Since the color developing reaction is activated by alkaline conditions, the photosensitive layer preferably contains bases such as amines or inorganic bases. Light — or heat-induced oxidation of the leuco dye 57 combined with cleavage of the N—X bond gave the indonaphthol — type cyan dye 58 (Scheme 20).
|
|
|
Table 3. Absorption Spectra of Quinone Derivatives26 30
|
|
|
|
|
co2r, cor
Scheme 20
Some quinones, having the ability to form intra — and/or intermolecular hydrogen bonds, exhibit high molecular hyperpolarizability and are third — order nonlinear optical (NLO) materials. Compound 39 has a %(3) of 5 x 10- 11 esu at 1.9 |am, and is a third-order NLO material.23 The optoelectric properties of quinoid compounds correlate with their structures in crystals or on thin films.23
 12 июля, 2015
12 июля, 2015  Malyar
Malyar 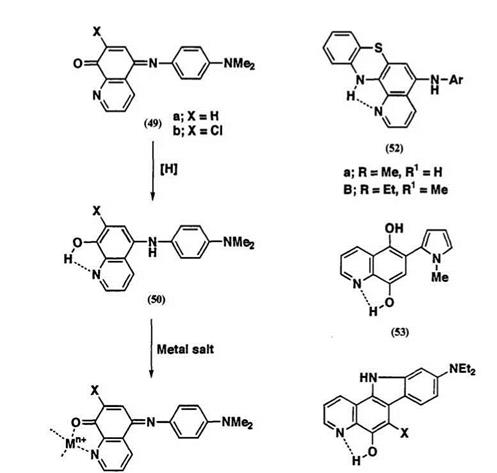
 Опубликовано в рубрике
Опубликовано в рубрике 