Benson et al. 177 first reported the synthesis of tetrazolium salts by the alkylation of disubstituted tetrazoles. While selectivity is a major problem, alkylation can still be considered a viable method for the preparation of
1,3,5- and 1,4,5-trisubstituted as well as 1,5 and 2,5-disubstituted tetrazolium salts.
Electronic effects of substituent, as well as reaction conditions (temperature, solvent) influence the ratio of isomers. The alkylation of 1-alkyl-5-aryl tetrazoles with dimethyl sulfate at room temperature proceeds at the
 |
expected 4-position, whereas at higher temperatures, the thermodynamically more stable 2,4-dialkyl 5-aryl isomer is obtained, possibly through the isomerization of the 1,4-dialkyl derivative.178-180,473 In the alkylation of 1-aryl-5-methyl tetrazole, the ratio of 3,5- and 4,5-dialkyl isomers changes with the nature of the alkylating agent.181 In contrast, in the alkylation of
1,5- dialkyl tetrazoles, the 1,3,5- to 1,4,5-isomer ratio is either closer to 1:1 or exclusively the 1,4,5-trialkyl isomer.182 1-Alkyl tetrazoles also undergo alkylation at the 4-position.183 2,5-Disubstituted tetrazoles are less reactive than the 1,5 isomers, but yield 1,3,5 isomers exclusively,180,183-187 whereas 1-aryl-5-(dialkylaminovinyl) tetrazoles (84) undergo alkylation at the 4- position exclusively.182 The alkylation of mesoionic tetrazoles takes place on the exocyclic heteroatom in good yields.188
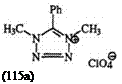 |
Synthesis of 1,4-Dimethyl-5-phenyltetrazolium perchlorate (115a). 180 1.92 g of 1-methyl-5-phenyltetrazole was added with stirring to 2.06 g of
dimethyl sulfate. The mixture was heated for 15 h at 35 °C, cooled, and poured into 20ml of ether and stirred. The ether layer was removed by decantation and the residue was washed with ether (4 x 20 ml), hexane (2 x 20 ml), and benzene (2 x 20 ml). The resulting solid was taken up in 10 ml of water and 1 ml of 57% perchloric acid was added. The product was filtered, washed with cold water, and recrystallized from isopropanol to yield
2.1 g of the tetrazolium salt (67%), mp 159—160°C.
Synthesis of 5-Ethoxy-1,3-diphenyltetrazolium tetrafluoroborate (111a). 188
1.2 g of 1,3-diphenyltetrazolium-5-olate was added to a solution of 1.0 g
|
|
of triethyl oxonium tetrafluoroborate in 20 ml of dichloromethane. After 16 h, ether was added and the precipitate collected. Recrystallization from acetone/light petroleum (bp 40—60 °C) gave 1.6 g of product (9%) mp 174°C.
 16 сентября, 2015
16 сентября, 2015  Malyar
Malyar 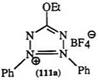
 Опубликовано в рубрике
Опубликовано в рубрике 