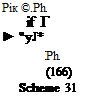 |
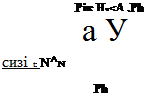 |
||
The alkylation of formazan anions (166) with methyl iodide yields the A-methylformazan (202) which cannot be prepared by direct methods (Scheme 31).4 However, formazans that contain hydroxyaryl (203) or heterocyclic groups (205) alkylate preferentially on these groups to yield the alkylation products 204349 and 206,346-348 respectively (Eqs. 24,25). Although formazans are resistant to acylation,334,350,351 it can be accom-
|
|
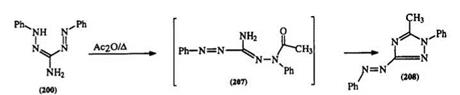
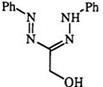
plished with acetic anhydride in the presence of zinc chloride.13’21’27’334 2-Amino-l,5-diphenylformazan (200) is also resistant to acylation with acetic anhydride under mild conditions; however, on heating, the tetrazole (208) is obtained, through acylation of the hydrazo nitrogen (207) (Scheme 32).28 By contrast C-hydroxyalkyl formazans (209) react with acetic anhydride to form the expected 0-acetyl derivative.351
|
Scheme 32 |
|
|
|
|
 4 октября, 2015
4 октября, 2015  Malyar
Malyar 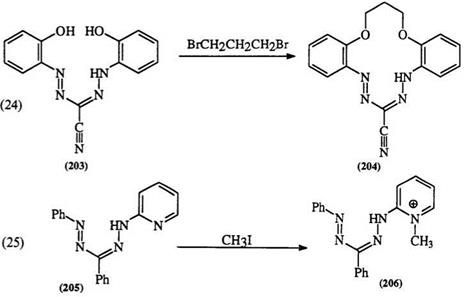
 Опубликовано в рубрике
Опубликовано в рубрике 