Introduction of an ethylene bridge between the meso-carbon atom and one of the diaminophenyl groups of a triarylmethane-type phthalide results in a considerable bathochromic shift, thus producing color formers exhibiting absorption in the near infrared region of the electromagnetic spectrum.
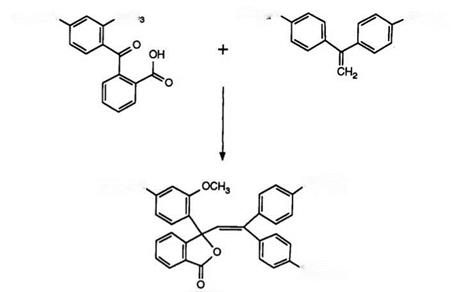 Scheme 9
Scheme 9
The preparation of such compounds was first described in 1975 in a patent87 encompassing a vast number of derivatives. Scheme 9 exemplifies the synthesis.
 |
Condensation of the benzophenone with the diarylaminoethylene takes place in acetic anhydride and in the original report 3-indolylarylamino- ethylenes were also described. In the meantime, a considerable number of variations have been reported, including the use of phenylarylaminoethy — lenes and also their synthesis from acetophenones and Grignard reagents.88 The use of diarylaminobenzophenonecarboxylic acids89 and also 3-carba- zolylphenyl analogues90 have been described.
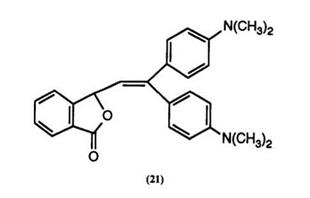
Reaction of 2-formylbenzoic acids with 1 ‘,1-bisdialkylaminoethylenes in acetic anhydride has been found91 to yield phthalides such as 21. These color formers are claimed to yield blue to green images, but have also been described92 as intermediates for the preparation of divinyl phthalides by a route identical to that described in Scheme 3, for which they are probably of more significance. (See Section 4.6.2.)
 3 августа, 2015
3 августа, 2015  Malyar
Malyar  Опубликовано в рубрике
Опубликовано в рубрике 