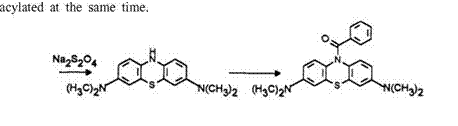
The standard procedure for the synthesis of leuco dyes related to benzoyl leuco Methylene Blue is straightforward. The one-pot synthesis is carried out in a two-phase water-toluene system. Methylene Blue is first dissolved in the aqueous phase and reduced with sodium dithionite under nitrogen and with stirring. The yellowish leuco is extracted into the organic phase where it is allowed to react with an acid chloride, the aqueous phase being made alkaline.
 |
Leuco Azure A or B is obtained by refluxing the dye in an acid anhydride in the presence of zinc powder wherein the dye is reduced and
|
|
Synthetic Method 1: 6-(dimethylamino)-3-(N-acetyl-N-methylamino)- 10-acetylphenothiazine 8a (procedure from US. Patent 4,652,643)3 A mixture of 9.0g of 6-(dimethylamino)-3-(methylamino)phenothiazin-5-ium chloride (Azure B), 150.0ml of acetic anhydride, and 10.0g of zinc dust was maintained at reflux temperature for approximately 4 hs. After the reaction mixture was cooled to ambient temperature, it was poured into ice water with stirring and 300ml of toluene was added. After stirring for approximately 30 min the toluene layer was separated and washed twice, once with tap water and once with saturated aqueous sodium chloride solution. The toluene was then distilled off at reduced pressure. The residue which remained was dissolved in ethyl acetate and separated into various components by subjecting the solution to column chromatography using silica gel as substrate. Elution with ethyl acetate yielded a white-colored solid.
Synthetic Method 2: 6-(dimethylamino) -3-[N-(4-methylphenylcar-
bonyl)-N-methylamino]-10-(4-methylphenylcarbonyl)-phenothiazine (8m) (procedurefrom U. S. Patent 4,652,643)3 The reaction vessel was purged of residual air with nitrogen and, while maintaining a nitrogen atmosphere, there was placed in the vessel 10.0g of Azure B, 500.0ml of water, and 500.0ml of toluene. With stirring, there was added to the resulting mixture 10.0 g of sodium carbonate and 15.0g of sodium dithionite. The resulting mixture was stirred for approximately 15 min at ambient temperature and the water layer was separated and discarded. To the toluene layer, 10.0 g of sodium dithionite was added and the resulting mixture was heated at reflux temperature until all of the water was azeotroped off. After the mixture had dried, it was cooled to approximately 70°C and 15.0g of disodium phosphate was added. To this mixture, there was added a solution of 20ml of 4-methylbenzoyl chloride dissolved in 30.0 ml of toluene. The reaction mixture was heated at reflux temperature for approximately 2_12 h. After cooling the resulting mixture to ambient temperature, 500ml of water and 15.0g of disodium phosphate were added. This mixture was then refluxed for approximately 30 min and then cooled to room temperature. The toluene layer was separated and saved and the water layer discarded. The toluene layer was washed twice, each time with 400ml of water, once with 400ml of aqueous saturated sodium carbonate solution, then with 400 ml of water and finally with 400ml of aqueous saturated sodium choride solution. All of the aqueous washes were discarded. The toluene layer was then
evaporated to dryness under reduced pressure. The residue was slurried in a mixture of 200ml of isopropyl alcohol, 100ml of water, and 20.0g of disodium phosphate at approximately 80°C for 10 min. After cooling, the solid was collected by filtration and dried to obtain 8.24 g of a white powder which melted at 220 to 224°C.
 16 июля, 2015
16 июля, 2015  Malyar
Malyar 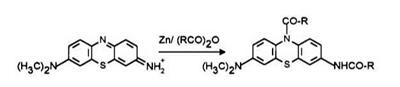
 Опубликовано в рубрике
Опубликовано в рубрике 