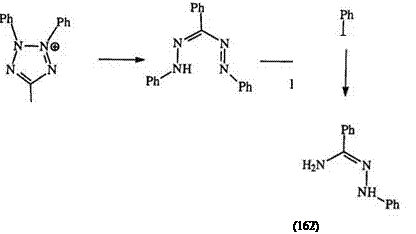
This is by far the most important reaction of tetrazolium salts and accounts for the bulk of their many applications. A large variety of reagents can reduce tetrazolium salts, e. g., 53 to formazans, e. g., 51. Ascorbic acid, hydrazine, and hydroxylamine have been recommended for the preparation of formazans from tetrazolium salts.245 Stronger reducing agents such as ammonium sulfide, sodium amalgam, sodium dithionite, and catalytic hydrogenation can further reduce the formazans to the amidrazones, e. g.,
 |
Scheme 24
162, through the hydrazidine intermediate (161) (Scheme 24).76,196 In symmetrically substituted tetrazoliums, as in 53, the amidrazones can be isolated. However, unsymmetrically substituted tetrazoliums lead to complex mixtures. It is claimed that irrespective of the reducing agent, the reduction proceeds cleanly to the formazan whenever the formazan can be precipitated during the reduction.244 As in photochemical reduction, the free radical intermediate (154) is proposed as the first step in the reduction as confirmed for many systems by ESR spectroscopy.226,261-264 The reduction of the commercially important bis tetrazolium salt (163) gives a red mono formazan (164) which then disproportionates to the blue formazan (165) and the bis tetrazolium salt (163) (Scheme 25).267-271 Th e reduction of mono and bis tetrazolium salts by reduced nicotinamide adenine dinucleotide, NADH, is very important in biochemistry and will be discussed in Section 7.6.2.
|
(165) |
 28 сентября, 2015
28 сентября, 2015  Malyar
Malyar 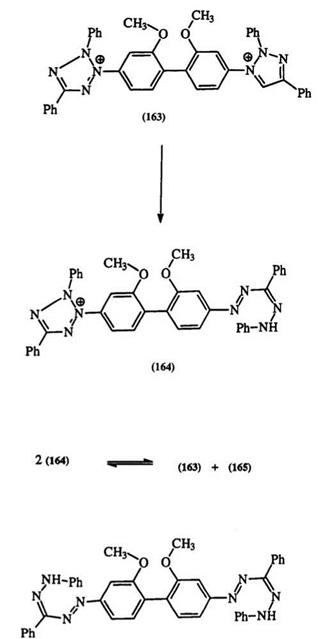
 Опубликовано в рубрике
Опубликовано в рубрике 