 |
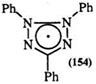
Solutions of tetrazolium salts, e. g., 53, have been reported to both become colored and bleached under the influence of both UV and visible light. Several workers have attributed this phenomenon to photoreduction to the corresponding formazan (51) and the formation of a fluorescent colorless compound (152) through photooxidation.240- 243 The reduction of 152 under UV or blue light to the intense green radical structure (153) has also been reported (Scheme 21).244 A one-electron reduction product (154) is proposed as a short-lived intermediate in the photoreduction.245
This reaction is influenced by solvent. Thus, in aqueous solutions 2,3,5- triphenyltetrazolium chloride yields 1,3,5-triphenylformazan (51), while in ethanol 152 is obtained. The mechanism of this reaction is not well understood.246 — 254
In a related manner, gamma radiolysis of tetrazolium salts also yields formazans.256,257 Pulse radiolysis in aqueous solutions leads to tetrazole
![]()
![]() (152)
(152)
Colorless, fluorescen
![]() Scheme 21
Scheme 21
radicals which can then disproportionate to formazan anions and tetra — zoliumsalts.258-260
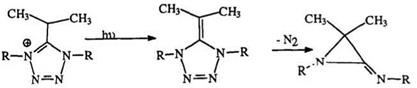 |
Unlike others, the 5-isopropyl tetrazoliums (155) undergo photodeprotonation to 156 which then yields the aziridimine (157) with the elimination of nitrogen (Scheme 22).254
|
(155) |
(156) |
(157) |
|
R= Alkyl or Aryl |
 |
Scheme 23
 26 сентября, 2015
26 сентября, 2015  Malyar
Malyar 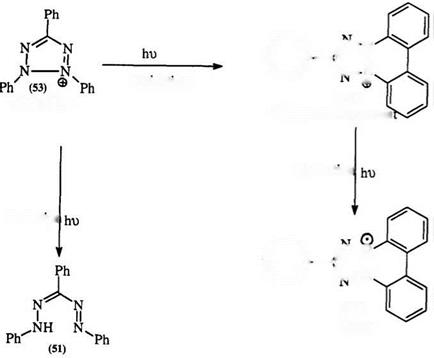
 Опубликовано в рубрике
Опубликовано в рубрике 