 |
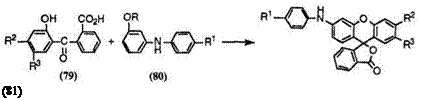
The reaction of keto acids (79) having no amino group with 3-alkoxy — diphenylamines (80) is used to synthesize 3 ‘-anilinofluorans (81), especially near-infrared-absorbing fluoran compounds (Eq. 6).
(6)
Table 7 shows melting points of a few near-infrared-absorbing fluoran compounds (81) thus prepared.
Preparation of 6′-[4-(4-Anilinoanilino)anilino]-2′-chloro-3′-methylflu — oran (81a). To concentrated sulfuric acid (10 g) was added 2-(5-chloro-2- hydroxy-4-methylbenzoyl)benzoic acid (3.4 mmol) followed by 4-anilino-4′- (3-methoxyanilino)diphenylamine (2.6 mmol). The mixture was stirred at room temperature for 24h, and poured into ice water (100ml). The precipitate was filtered off, washed with water, and then refluxed with a mixture of toluene (150 ml) and sodium hydroxide (20 g) dissolved in water (150ml) for 1 h. The toluene layer was separated, washed with hot water, and concentrated. The residue was then column chromatographed on silica gel to give 6′-[4-(4-anilinoanilino)anilino]-2′-chloro-3′-methylfluoran in 43% yield as a grayish white powder, mp 202-203 °C.
|
|
|
81 |
R1 |
R2 |
R3 |
о 0 % |
|
a |
4-C6H5NHC6H4NH |
CH3 |
Cl |
202-203 |
|
b |
4-C6H5NHC6H4NH |
H |
CH3 |
133-136 |
|
c |
4-(CH3)2C6H4NH |
H |
CH3 |
199-202 |
 1 сентября, 2015
1 сентября, 2015  Malyar
Malyar 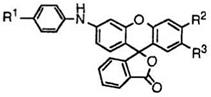
 Опубликовано в рубрике
Опубликовано в рубрике 