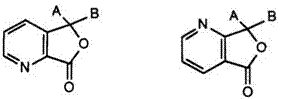
The possibility of replacing all three phenyl rings in the triarylmethane lactone structure by heterocycles has also been exploited. The first compound to be described83 was the 3,3-bisindolyl-7-azaphthalide (18). This
|
(18) |
compound was obtained directly by reaction of 2 mol of 1,2-dimethylindole with pyridine-2,3-dicarboxylic acid anhydride in acetic anhydride. However, the unambiguity of this 7-azaisomer would seem somewhat doubtful since a later report84 describes the preparation of 4- and 7-azaisomeric mixtures by sequential reaction of two differing 1,2-disubstituted indoles with the anhydride. Further examples of asymmetric 7-azaphthalides with indoles and benzindoles have also been cited.85 These color formers give red to purple images with clay and phenolic resin developers with good light stability.
Mixtures of 4- and 7-azaphthalides carrying a wide variety of heterocycles such as carbozolyl, pyrrolyl, acridinyl, phenothiazinyl, thianaph — thenyl, and thienyl as well as indolyl of the general formulas 19 and 20 have also been described.86 Table 1 illustrates the variety of hues available with such compounds.
4-Azaphthalides carrying indolyl and indolizinyl substituents have been shown65 to produce blue color formers, while the combination of indoles
|
Table 1. Hues Available with Mixtures of 4- and 7-Azaphthalides Carrying Various Heterocycles
|
 |
with imidazo[ 1,2-a]pyridines results in red images with clay developers.66 Pyrazine-2,3-dicarboxylic acid anhydride has been found85 to react analogously, producing red color formers on sequential reaction with benzindoles and pyrroles, while indoles and indolizines produce blue derivatives.65 Finally, quinoxaline-2,3-dicarboxylic acid anhydride has frequently been reacted with indoles, and the intermediate ketoacid then condensed with benzindoles,82 indolizines,65 and imidazo[ 1,2-a]pyridines to produce red, blue-black, and orange color formers, respectively.
 2 августа, 2015
2 августа, 2015  Malyar
Malyar 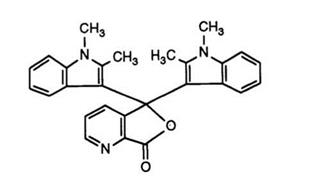
 Опубликовано в рубрике
Опубликовано в рубрике 