Introduction of two diaminophenylethylene moieties at the 3-position of the phthalide ring naturally also produces color formers exhibiting infrared absorption. As in Section 4.6.1, the first report93 encompasses a vast number of compounds such as 22 which was prepared by treating phthalic anhydride with 2 mol of 1,1-bisdimethylaminophenylethylene in acetic anhydride.
|
|
(22)
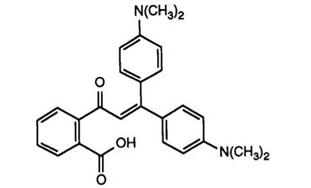
Reaction proceeds via the intermediate keto acid, but, in practice, a one-pot procedure was employed. Substitution of the phthalide ring by chlorine or bromine atoms and replacement of the dimethylaminophenyl groups by tetrahydroquinoline or a 4-pyrrolidinylphenyl residue has been reported94 to yield color formers possessing high resistance to heat, moisture, and light. Similar effects have also been claimed95,96 for compounds in which one dialkylamino group was replaced by an alkoxy substituent, the preparation of the required diarylethylenes from acetophenones and Grig — nard reagents also being described. Tetrachlorophthalic anhydride has also been reacted97 with 1-(3-dimethylaminophenyl)-1-(4-dimethylamino — phenyl)ethylene to give a color former exhibiting a remarkably high absorption (935 nm), in combination with a phenolic resin developer. As mentioned in Section 4.6.1, phthalides such as 21 are useful intermediates since oxidation, for example with 3-nitrobenzenesulfonic acid, yields benzo — phenones (23), which may then be reacted with a second different ethylene to give asymmetric bisethylenyl phthalides.98
|
|
Finally, bisindolylethylenes have also been reacted with tetrahaloph — thalic anhydrides to yield color formers showing absorption in the near infrared.99 The chief advantage over the diarylethylenes is the availability of the starting materials. The bisindolylethylenes may be prepared in situ by reaction of an indole with acetyl chloride and then converted directly to the phthalide without isolation.
 3 августа, 2015
3 августа, 2015  Malyar
Malyar 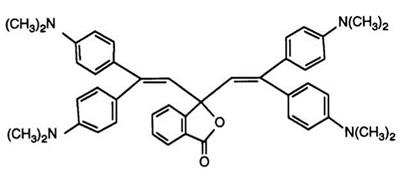
 Опубликовано в рубрике
Опубликовано в рубрике 